Adagene (ADAG) Gets FDA Nod for Cancer Study on ADG126 Combo
Adagene Inc. ADAG announced that it has received clearance from the FDA to initiate a clinical study evaluating its anti-CTLA-4 monoclonal antibody (mAb), ADG126 in combination with anti-PD-1 antibody, pembrolizumab for treating patients with advanced/metastatic solid tumors.
The company will dose the first patients in the global phase Ib/II study – ADG126-P001 – soon.
The study will investigate the safety and tolerability of the combo of ADG126 + pembrolizumab in patients with advanced/metastatic solid tumors at multiple sites in the United States and the Asia Pacific. The study will also help determine the recommended phase II dose for the combo of ADG126 plus pembrolizumab.
Per the company, ADG126 is the first SAFEbody candidate to enter into a combination clinical study. Additionally, a combination cohort of ADG126 with the anti-PD-1 therapy, toripalimab, is also being initiated in Australia.
Shares of Adagene have plunged 40% so far this year compared with the industry’s decline of 19.1%.
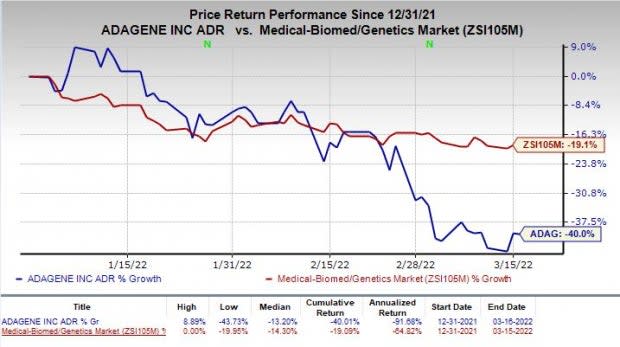
Image Source: Zacks Investment Research
We note that pembrolizumab is an anti-PD-1 therapy developed by pharma giant, Merck MRK. The same is marketed under the brand name Keytruda by Merck.
Adagene has a clinical collaboration and supply agreement with Merck to develop ADG126 in combination with pembrolizumab for advanced/metastatic solid tumors.
Keytruda is currently the biggest top-line driver for Merck. Keytruda is already approved for treating many cancers globally and is continuously growing and expanding into new indications and markets worldwide. In 2021, Merck recorded $17.2 billion in sales from Keytruda.
Apart from ADG126, Adagene has two product candidates in its pipeline, an anti-CD137 agonist called ADG106 and another anti-CTLA-4 mAb product candidate, ADG116. The company also has clinical collaborations and supply agreements with Merck for each of these clinical candidates.
Last November, the FDA cleared the investigational new drug (IND) application, which sought approval for initiating a clinical study on ADG116 in combination with pembrolizumab for treating patients with advanced/metastatic solid tumors.
Meanwhile, ADG106 is being evaluated in combination with pembrolizumab for addressing advanced or metastatic solid and/or hematological malignancies.
Zacks Rank & Stocks to Consider
Adagene currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the biotech sector are Vertex Pharmaceuticals Incorporated VRTX and Kaleido Biosciences, Inc. KLDO, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Vertex’s earnings estimates have been revised 8.4% upward for 2022 over the past 60 days. VRTX stock has gained 13.2% year to date.
Vertex’s earnings have surpassed estimates in each of the trailing four quarters.
Kaleido Biosciences’ loss per share estimates have narrowed 23% for 2022 over the past 60 days.
Earnings of Kaleido Biosciences surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Kaleido Biosciences, Inc. (KLDO) : Free Stock Analysis Report
Adagene Inc. Sponsored ADR (ADAG) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
