Amgen's Aimovig Reduces Episodic Migraine in 30% Patients
Amgen Inc. AMGN has announced full results from the phase IIIb study on its anti-CGRP receptor antibody, Aimovig (erenumab), which once again demonstrates a significant reduction in migraine frequency. The company is looking to get Aimovig (140mg) approved for treatment of episodic migraine in patients with a previous experience of two to four preventive treatment failures due to lack of efficacy or intolerable side effects.
The phase III LIBERTY study randomized 246 patients with the aforementioned indication to receive Aimovig 140 mg or placebo for 12-weeks. Data from the study demonstrated reduction in episodic migraines by at least half in 30% of Aimovig treated patients with multiple treatment failures compared with 14% of patients treated with placebo.
Moreover, over 97% of Aimovig patients completed the double-blind phase of the LIBERTY trial. No adverse events were found that might lead to discontinuation of treatment in the Aimovig group. Outcomes from the study will be presented at the 70th annual meeting of the American Academy of Neurology in Los Angeles.
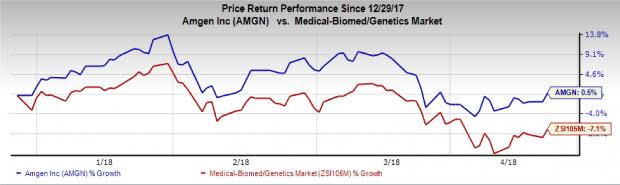
Shares of Amgen increased more than 2% on Apr 17 following the news release. The stock has gained 0.5% so far this year against the industry's 7.1% decline.
We remind investors that in January this year, the company announced that Aimovig met its primary endpoint in the LIBARTY study with significantly more patients under Aimovig’s dosage experiencing at least a 50% reduction from baseline in their monthly migraine days as compared to placebo. The study met its secondary endpoint as well.
Notably, Amgen is developing Aimovig in collaboration with Novartis AG NVS. The companies are focused on developing and commercializing the treatments for migraine and Alzheimer's disease. If approved, Amgen and Novartis will co-commercialize Aimovig in the United States. However, Amgen retains commercialization rights in Japan while Novartis has rights in Europe, Canada and the Rest of World.
Importantly, the candidate is under review in the United States for the same indication with a response expected in May 17, 2018. Notably, the candidate is the first investigational therapy targeting the CGRP pathway to having received a regulatory filing acceptance from the FDA for the given indication.
Per the company, around 10 million people in the United States are affected by migraine. Among them, approximately 3.5 million are on a preventive therapy with 80% likely to discontinue the treatment within a year. Thus, approval of the drug will address the hugely unmet need of patients suffering the disease in the country.
However, several companies, namely Teva Pharmaceutical TEVA and Eli Lilly LLY among others are developing migraine treatments targeting CGRP.
Amgen Inc. Price
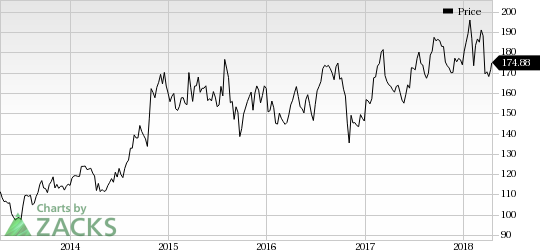
Amgen Inc. Price | Amgen Inc. Quote
Zacks Rank
Amgen carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Can Hackers Put Money INTO Your Portfolio?
Earlier this year, credit bureau Equifax announced a massive data breach affecting 2 out of every 3 Americans. The cybersecurity industry is expanding quickly in response to this and similar events. But some stocks are better investments than others.
Zacks has just released Cybersecurity! An Investor’s Guide to help Zacks.com readers make the most of the $170 billion per year investment opportunity created by hackers and other threats. It reveals 4 stocks worth looking into right away.
Download the new report now>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Amgen Inc. (AMGN) : Free Stock Analysis Report
Teva Pharmaceutical Industries Ltd. (TEVA) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research