Apellis (APLS) Down on Mixed Data From Studies on GA Candidate
Shares of Apellis Pharmaceuticals, Inc. APLS were down 37.2% on Friday after the company announced top-line data from two phase III studies, namely, DERBY and OAKS, which is evaluating its targeted C3 therapy, pegcetacoplan for treating adult patients with geographic atrophy (“GA”) secondary to age-related macular degeneration.
In fact, the stock has plunged 38.9% so far this year against the industry’s rise of 0.8%.
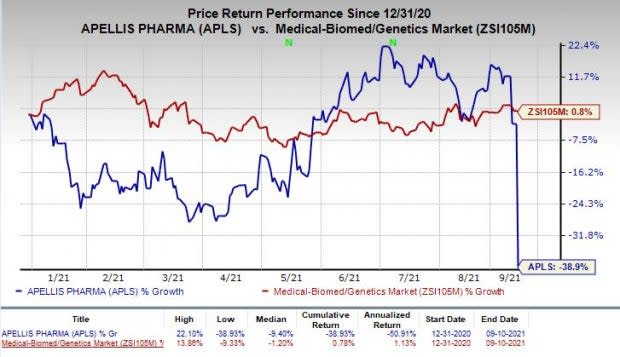
Image Source: Zacks Investment Research
Data from the OAKS study showed that monthly and every-other-month treatment with pegcetacoplan led to a significant reduction of 22% of 16%, respectively, in GA lesion growth as compared to pooled sham at 12 months – the primary endpoint.
Data from the DERBY study showed a reduction of 12% and 11%, respectively, with monthly and every-other-month treatment with pegcetacoplan, as compared to pooled sham at 12 months. The study did not meet the primary endpoint.
Pegcetacoplan was well tolerated across both studies and demonstrated a favorable safety profile. Data from the combined DERBY and OAKS studies showed that monthly and every-other-month treatment with pegcetacoplan reduced GA lesion growth by 17% and 14%, respectively.
Despite the mixed results, Apellis believes that pegcetacoplan has the potential to become the first treatment for GA, a leading cause of blindness. The company plans to submit a new drug application to the FDA for pegcetacoplan to treat GA in the first half of 2022.
Per the company, pegcetacoplan is the first investigational therapy to significantly slow the progression of GA in a phase III study.
An estimated one million people are affected by GA in the United States. Hence, if successfully developed for the given indication and upon potential approval, pegcetacoplan can become a new treatment option for such patients. Currently, there is no approved therapy for GA.
We remind investors that in May 2021, the FDA approved Empaveli (pegcetacoplan) as a monotherapy treatment for adult patients suffering from paroxysmal nocturnal hemoglobinuria (“PNH”). A rare blood disorder, PNH is associated with abnormally low hemoglobin levels as the disease destroys red blood cells.
Empaveli is approved for treatment-naïve patients as well as those switching from Alexion’s [now part of AstraZeneca’s AZN] C5 inhibitor therapies for PNH, namely Soliris (eculizumab) and Ultomiris (ravulizumab).
Zacks Rank & Stocks to Consider
Apellis currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector include Spero Therapeutics, Inc. SPRO and Corvus Pharmaceuticals, Inc. CRVS, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Spero Therapeutics’ loss per share estimates have narrowed 8.2% for 2021 and 10.6% for 2022, over the past 60 days.
Corvus Pharmaceuticals’ loss per share estimates have narrowed 24.4% for 2021 and 21.4% for 2022, over the past 60 days.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Corvus Pharmaceuticals, Inc. (CRVS) : Free Stock Analysis Report
Spero Therapeutics, Inc. (SPRO) : Free Stock Analysis Report
Apellis Pharmaceuticals, Inc. (APLS) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
