Axsome's NDA for Depressive Disorder Drug on Track in Q420
Axsome Therapeutics, Inc. AXSM announced that it has completed a successful pre-new drug application (NDA) meeting with the FDA on one of its lead pipeline candidates, AXS-05, for the treatment of major depressive disorder (MDD). The company remains on track to submit the planned NDA in the fourth quarter of 2020.
Acceptance of the final NDA will be subject to the regulatory body’s review of the complete filing. Notably, in March 2019, the FDA granted a Breakthrough Therapy designation to AXS-05 for MDD. Per the company, the Breakthrough Therapy status makes AXS-05 eligible for priority review.
Per data announced last December, AXS-05 met the primary endpoint and significantly improved symptoms of depression in the phase III GEMINI study on MDD.
Importantly, data from both the GEMINI and ASCEND studies showed that treatment with AXS-05 led to a rapid, substantial and statistically significant reductions in depressive symptoms compared to the control arm.
According to management, positive results from the company’s GEMINI study on MDD along with the previously-completed ASCEND study will be sufficient to support the filing of the NDA for AXS-05 to address the given indication.
If successfully developed and upon potential approval, AXS-05 will be the first orally administered N-methyl-D-aspartate (NMDA) receptor antagonist to get the nod for the treatment of MDD.
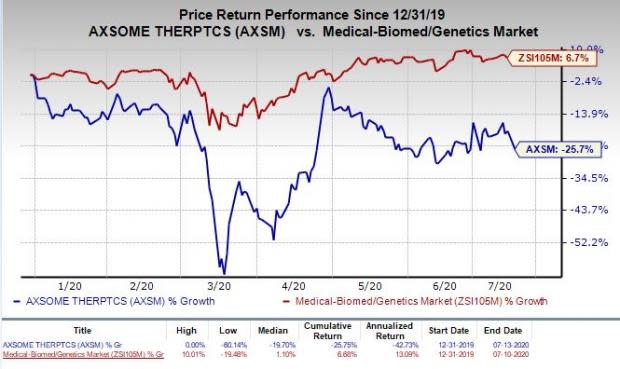
Despite the progress in the pre-NDA meeting, shares of Axsome were down 7.6% following the above development on Monday. In fact, so far this year, the stock has declined 25.7% against the industry’s increase of 6.7%.
AXS-05 is a novel, oral, investigational non-competitive NMDAreceptor antagonist with multimodal activity under development for treating central nervous system (CNS) disorder. The candidate is also being studied for agitation associated with Alzheimer's disease (AD).
Last month, the FDA granted a Breakthrough Therapy tag to AXS-05 for treating agitation associated with AD. It was based on the recent positive results from the pivotal phase II/III ADVANCE-1 study, which evaluated AXS-05 for the given indication.
Meanwhile, AXS-05 is also being evaluated in the phase III STRIDE-1 study to address patients with treatment-resistant depression (TRD).
Zacks Rank & Stocks to Consider
Axsome currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the healthcare sector include Emergent Biosolutions Inc. EBS, BioMarin Pharmaceutical Inc. BMRN and Unum Therapeutics Inc. UMRX, all presently sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Emergent’s earnings estimates have been revised 26.7% upward for 2020 and 29.8% for 2021 over the past 60 days. The stock has soared 69.2% year to date.
BioMarin’s earnings estimates have moved 3.3% north for 2020 and 13.4% for 2021 over the past 60 days. The stock has surged 47.1% year to date.
Unum Therapeutics’ loss per share estimates have narrowed 46% for 2020 and 82.8% for 2021 over the past 60 days.The stock has skyrocketed 355.4% year to date
5 Stocks Set to Double
Each was hand-picked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2020. Each comes from a different sector and has unique qualities and catalysts that could fuel exceptional growth.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
BioMarin Pharmaceutical Inc. (BMRN) : Free Stock Analysis Report
Emergent Biosolutions Inc. (EBS) : Free Stock Analysis Report
Axsome Therapeutics, Inc. (AXSM) : Free Stock Analysis Report
Unum Therapeutics Inc. (UMRX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
