Aytu BioPharma's (AYTU) AR101 Gets FDA's Orphan Drug Status
Aytu BioPharma, Inc. AYTU announced that the FDA has granted an Orphan Drug designation to its investigational pipeline candidate, AR101 (enzastaurin), for the treatment of Ehlers-Danlos Syndrome. The treatment of vascular Ehlers-Danlos Syndrome (“VEDS”) is within the scope of this designation.
The Orphan Drug designation is granted by the FDA to a drug or biologic intended to treat a rare disease or condition, which generally includes a disease or condition that affects fewer than 200,000 individuals in the United States. The designation also includes incentives, including financial aid for clinical testing, and seven-year marketing exclusivity in the event of regulatory approval.
Aytu BioPharma plans to begin the single pivotal PREVEnt study in the first half of 2022, evaluating AR101 to treat patients with VEDS, a rare genetic disorder. Currently, there is no FDA-approved therapy to address the given indication. The primary endpoint of the study is to see the reduction in fatal or non-fatal arterial events, like ruptures, dissections and pseudo-aneurisms.
The company is also seeking Orphan Drug designation for AR101 from the European Medicines Agency.
Shares of Aytu BioPharma have plunged 68.9% so far this year compared with the industry’s decline of 20.5%.
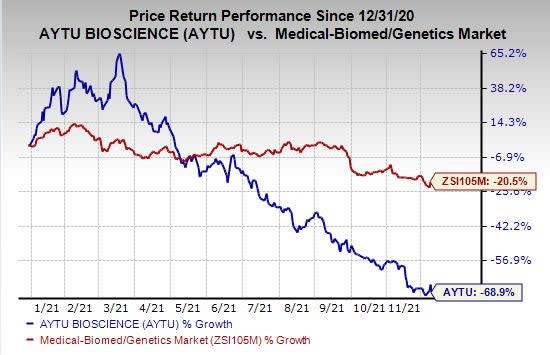
Image Source: Zacks Investment Research
We remind investors that Aytu BioPharma reports revenues from the prescription therapeuticsdivision and the consumer health division.The company's prescription products – Adzenys XR-ODT and Cotempla XR-ODT are indicated to treat attention deficit hyperactivity disorder (ADHD) and other pediatric conditions.
Both these products have witnessed a strong uptake and growth in prescriptions during the first quarter of fiscal year 2022 (ended Sep 30, 2021). During the same time, the company’s consumer health division also grew on a year-over-year basis.
Aytu BioPharma also markets several other pediatric products, including ZolpiMist (zolpidem tartrate oral spray), which is approved for short-term treatment of insomnia.
Zacks Rank & Stocks to Consider
Aytu BioPharma currently carries a Zacks Rank #4 (Sell).
Better-ranked stocks in the biotech sector include Sarepta Therapeutics, Inc. SRPT, Editas Medicine, Inc. EDIT and vTv Therapeutics Inc. VTVT, all carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Sarepta Therapeutics’ loss per share estimates have narrowed 31.3% for 2021 and 26% for 2022, over the past 60 days.
Earnings of Sarepta Therapeutics have surpassed estimates in two of the trailing four quarters, and missed the same on the other two occasions.
Editas Medicine’s loss per share estimates have narrowed 11.2% for 2021 and 4.6% for 2022, over the past 60 days.
Editas Medicine’s earnings have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions.
vTv Therapeutics’ loss per share estimates have narrowed 21.7% for 2021 and 2.9% for 2022, over the past 60 days.
vTv Therapeutics’ earnings have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
vTv Therapeutics Inc. (VTVT) : Free Stock Analysis Report
Editas Medicine, Inc. (EDIT) : Free Stock Analysis Report
Aytu BioPharma Inc. (AYTU) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
