Biogen (BIIB) Inks Licensing Deal for Implantable Medical Device
Biogen Inc. BIIB announced that it has entered into a license and collaboration agreement with Massachusetts-based privately held biotech, Alcyone Therapeutics to develop the latter’s ThecaFlex DRx system, an implantable medical device designed for subcutaneous delivery of antisense oligonucleotide (ASO) therapies into the intrathecal space.
Per the agreement, Biogen will make an upfront payment of $10 million to Alcyone for getting an exclusive global license to develop ThecaFlex DRx system in spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS) and a co-exclusive global license for an unnamed indication.
Alcyone will also be eligible to receive up to $41 million from BIIB as potential milestone payments upon achieving certain development and commercial milestones.
With this deal, Biogen is looking to leverage the ThecaFlex DRx system for improving treatment experience and getting access to patients suffering from neurological disorders, such as SMA and ALS.
The FDA has granted Breakthrough Device Designation to the ThecaFlex DRx system. It has also received a CE Mark in Europe.
Both companies will jointly develop the ThecaFlex DRx system for ASO therapies, while Alcyone will be solely responsible for manufacturing and commercializing the same. The novel medical device will be initially evaluated with Biogen’s Spinraza (nusinersen) in SMA, which is likely to pave the way for the company’s broader portfolio of investigational ASO therapies.
Shares of Biogen have rallied 14.1% in the past year against the industry’s decline of 17.7%.
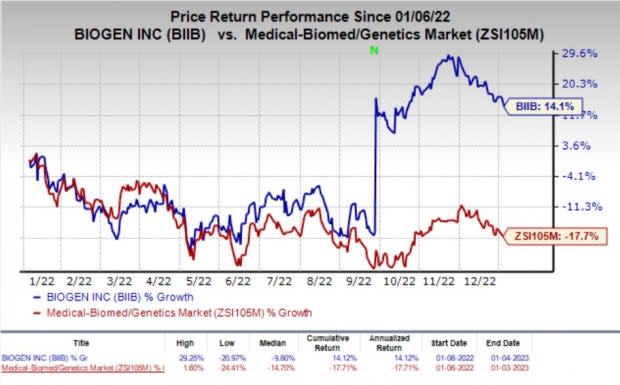
Image Source: Zacks Investment Research
Biogen’s Spinraza has consolidated its position in the neurological disease market with the drug being the first treatment for SMA to be approved in the United States. Biogen has expanded its collaboration with Ionis Pharmaceuticals IONS to identify up to three gene targets for the treatment of SMA as well as a broad range of neurological diseases.
Biogen and Ionis’ Spinraza has now become the standard-of-care treatment for treating SMA. While Biogen is responsible for Spinraza’s sales, Ionis receives royalties on the same.
However, Spinraza sales are being hurt due to a decrease in demand as a result of increased competition from Novartis’ NVS new SMA treatment, Zolgensma and Roche RHHBY and PTC Therapeutics’ Evrysdi (risdiplam) and unfavorable pricing.
Novartis’ Zolgensma was approved in May 2019 while Roche/PTC Therapeutics’ Evrysdi was approved by the FDA in August 2020.
Zacks Rank
Biogen currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
