Biotech Stock Roundup: MACK Up on Study Data, MRNA Offers Vaccine Update & More
Drug approvals and other regulatory news are in focus in the biotech sector. Updates on booster doses of COVID-19 vaccines are also in the spotlight with the approaching winter season, which is likely to see a spike in the infection rate.
Recap of the Week’s Most Important Stories:
Merrimack Surges on Positive Data by Partner: Shares of Merrimack Pharmaceuticals, Inc. MACK skyrocketed after partner Ipsen announced that the that phase III NAPOLI 3 trial of Onivyde (irinotecan liposome injection) plus 5 fluorouracil/leucovorin and oxaliplatin (NALIRIFOX regimen) was successful. The trial met its primary goal. Data from the study demonstrated clinically meaningful and statistically significant improvement in overall survival compared to nab-paclitaxel plus gemcitabine in 770 previously untreated patients with metastatic pancreatic ductal adenocarcinoma (mPDAC). Further, the study met its key secondary endpoint of progression-free survival with a safety profile consistent with the previous study.
Investors cheered the data as Merrimack is entitled to receive up to $450 million in contingent milestone payments related to its sale of Onivyde to Ipsen S.A. in April 2017. These payments are contingent upon FDA approval of Onivyde for certain additional clinical indications. The drug is already approved by the FDA in combination with fluorouracil (5-FU) and leucovorin (LV) for the treatment of patients with metastatic adenocarcinoma of the pancreas after disease progression following gemcitabine-based therapy.
Moderna Vaccine Meets Goals: Moderna MRNA announced that the phase II/III study evaluating both of its mRNA-based bivalent Omicron-targeting COVID-19 vaccines, mRNA-1273.222 and mRNA-1273.214, achieved their study goals. Shares gained on the same. mRNA-1273.222 induced significantly higher neutralizing antibody titers against BA.4/BA.5 compared to a booster dose of the original COVID vaccine, Spikevax (mRNA-1273), in phase II/III study in more than 500 adults. The bivalent vaccines also met non-inferiority immunogenicity criteria to the original strain. While mRNA-1273.222 contains the spike protein in the BA.4/5 Omicron subvariants, mRNA-1273.214 contains the spike protein in the BA.1 Omicron subvariant.
An exploratory data analysis also revealed that both the bivalent vaccines demonstrated robust neutralizing activity against BQ.1.1, an emerging COVID-19 variant. The result from this analysis confirmed that the bivalent vaccines are effective against new and evolving variants. However, the neutralizing activity of these vaccines against the BQ.1.1 variant fell 5-fold in titers compared with the BA.4/5 variants.
Seagen’s Adcetris Label Expansion: Seagen SGEN announced that the FDA approved its lead cancer drug, Adcetris (brentuximab vedotin), for a new indication. Adcetris is now approved in combination with doxorubicin, vincristine, etoposide, prednisone and cyclophosphamide for treating pediatric patients aged two years and above with previously untreated high-risk classical Hodgkin lymphoma (cHL). The approval was based on a phase III study (AHOD1331) that demonstrated a 59% reduction in risk of disease progression or relapse, second malignancy or death versus standard of care.
Seagen currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
BrainStorm Provides Regulatory Update: BrainStorm Cell Therapeutics Inc. BCLI announced that the company has received a refusal to file a letter from the FDA regarding its new biologics license application (BLA) for NurOwn for the treatment of amyotrophic lateral sclerosis (ALS). The regulatory body has indicated that the company can request a Type A meeting to discuss the content of the refusal to file letter. BrainStorm holds the rights to clinical development and commercialization of the NurOwn technology platform used to produce autologous MSC-NTF cells through an exclusive, worldwide licensing agreement. The phase III NurOwn trial was a multi-center, placebo-controlled, randomized, double-blind trial designed to evaluate the safety and efficacy of repeat doses of NurOwn in 189 ALS participants
Brainstorm intends to request a Type A meeting with the FDA for the same. As part of the Type A meeting, the company plans to discuss with the FDA a path to an FDA Advisory Committee meeting.
Performance
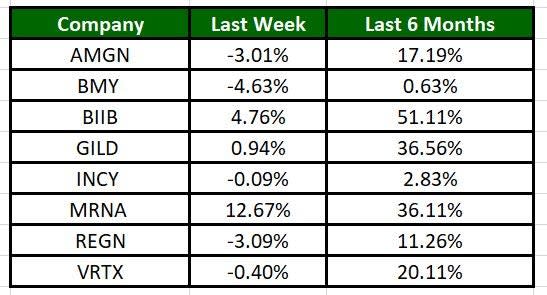
Image Source: Zacks Investment Research
The Nasdaq Biotechnology Index gained 3.12% in the past five trading sessions. Among the biotech giants, Moderna has gained 12.67% during the period. Over the past six months, shares of Biogen have soared 51.11%. (See the last biotech stock roundup here: Biotech Stock Roundup: REGN, AMGN, MRNA’s Q3 Earnings, VERV Down on Update)
What's Next in Biotech?
Stay tuned for other updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Seagen Inc. (SGEN) : Free Stock Analysis Report
Merrimack Pharmaceuticals, Inc. (MACK) : Free Stock Analysis Report
Brainstorm Cell Therapeutics Inc. (BCLI): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
