Biotech Stock Roundup: MRNA's Vaccine Update, GILD's CAR T Therapy Label Expansion
The biotech sector has been in focus last week with important regulatory and pipeline updates. This apart, updates on additional doses of COVID-19 vaccines and antibody treatments continue to grab the limelight.
Recap of the Week’s Most Important Stories:
Moderna’s Vaccine Update: Moderna MRNA announced that the European Medicines Agency (“EMA”) has authorized a third dose of its COVID-19 vaccine (Spikevax) given at least 28 days after the second dose to severely immunocompromised individuals 12 years of age or older. Data from a recent double-blind, randomized controlled study among 120 individuals who had undergone solid organ transplant procedures (heart, kidney, kidney-pancreas, liver, lung, or pancreas) showed that a third dose improved immune response compared to placebo in this population. The dose was generally well tolerated.
Gilead’s CAR T- Cell Therapy Label Expansion: Gilead Sciences, Inc.’s GILD Kite announced that it obtained FDA approval for another indication for its CAR T-cell therapy, Tecartus. It has been approved for the treatment of adult patients (18 years and older) with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). The approval is based on results from ZUMA-3, a global, multicenter, single-arm, open-label study in which 65% of the evaluable patients (n=54) achieved complete remission (CR) or CR with incomplete hematological recovery (CRi) at a median actual follow-up of 12.3 months. It is already approved for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL). Tecartus is also currently under review in the European Union and the United Kingdom for the treatment of adult patients with relapsed or refractory B-cell precursor ALL.
Earlier, Kite submitted a supplemental biologics license Aapplication (sBLA) to the FDA for Yescarta (axicabtagene ciloleucel) to expand its current indication to include the treatment of adults with relapsed or refractory large B-cell lymphoma (LBCL) in the second-line setting.
Regeneron’s Antibody Cocktail Data: Regeneron Pharmaceuticals, Inc. REGN announced that a phase II/III randomized, double-blind, placebo-controlled study evaluating experimental antibody cocktail, REGEN-COV (casirivimab and imdevimab), in patients hospitalized with COVID-19 met its primary endpoint.
The study results showed that REGEN-COV significantly reduced viral load in patients hospitalized with COVID-19 who entered into the trial without having mounted their own antibody response (seronegative) and required low-flow or no supplemental oxygen. REGEN-COV significantly reduced viral load within seven days of treatment. Patients who received REGEN-COV in this study experienced a 36% reduced risk of dying within 29 days of treatment, and in patients who were seronegative when they entered the trial, the risk was reduced by 56%. The FDA is currently reviewing the request to add treatment in hospital settings to REGEN-COV authorization.
Regeneron currently sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
BioNTech Doses First Patient in Cancer Vaccine Study: BioNTech SE BNTX announced that it has dosed the first patient in a phase II study of its individualized mRNA cancer vaccine BNT122 (autogene cevumeran, RO7198457). The open-label phase II study is investigating autogene cevumeran in stage II/III colorectal cancer patients after surgical resection of their tumor and completion of adjuvant chemotherapy. The study has been initiated in the United States, Germany, Spain, and Belgium. It plans to enroll about 200 patients to evaluate the efficacy of BNT122 compared to watchful waiting after surgery and chemotherapy. The primary endpoint for the study is disease-free survival (DFS). Secondary objectives include relapse-free survival (RFS), overall survival (OS) and safety.
Separately, BioNTech and partner Pfizer PFE announced that Committee for Medicinal Products for Human Use (“CHMP”) of the EMA issued a positive opinion on the administration of the companies’ COVID-19 vaccine as a booster dose at least six months after the second dose in individuals 18???years of age and older.
Regulatory Updates From Bristol-Myers: Bristol Myers BMY announced that the EMA has validated its Marketing Authorization Application (MAA) for experimental candidate mavacamten. The MAA is seeking approval for this first-in-class cardiac myosin inhibitor for the treatment of patients with obstructive hypertrophic cardiomyopathy (obstructive HCM). With the validation, EMA’s centralized procedure with CHMP’s assessment begins.
The EMA also validated its MAA for the fixed-dose combination of its LAG-3-blocking antibody relatlimab and Opdivo for the first-line treatment of adult and pediatric patients (12 years and older and weighing at least 40 kg) with advanced (unresectable or metastatic) melanoma.
Performance
Medical - Biomedical and Genetics Industry 5YR % Return

Medical - Biomedical and Genetics Industry 5YR % Return
The Nasdaq Biotechnology Index has lost 3.75% in the past five trading sessions. Among the biotech giants, Moderna has lost 13.56% during the period. Over the past six months, shares of Moderna have soared 155.65%. (See the last biotech stock roundup here: Biotech Stock Roundup: XLRN Up on Takeover Rumor, NVAX, BIIB Offer Updates & More).
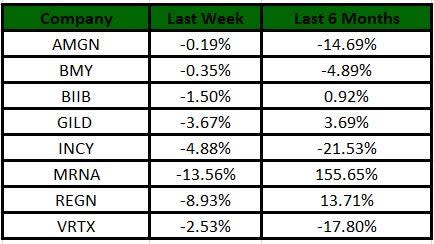
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for more pipeline and regulatory updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
