Biotech Stock Roundup: REGN Provides COVID-19 Updates, MYOV Partners With PFE & More
Quite a few regulatory and pipeline updates grabbed the spotlight in the biotech sector in the past week. Collaborations again took center stage in the sector. Meanwhile, COVID-19 vaccine updates gained more significance in the sector with each passing day as the pandemic registers a spike again.
Recap of the Week’s Most Important Stories:
Regeneron Announces Positive Data on Cocktail: Regeneron Pharmaceuticals, Inc. REGN announced encouraging initial data from an ongoing phase I/II/III study of its antibody cocktail, casirivimab and imdevimab, in hospitalized COVID-19 patients requiring low-flow oxygen. Casirivimab and imdevimab is a cocktail of two monoclonal antibodies (also known as REGN10933 and REGN10987, respectively) and was designed specifically to block the infectivity of SARS-CoV-2, the virus that causes COVID-19. The analysis was prospectively designed to focus on patients who had not yet developed their own immune response to SARS-CoV-2 (i.e., did not have antibodies at baseline: seronegative). The initial analysis was conducted to determine if there was sufficient efficacy in these patients to warrant the continuation of the trial (i.e., futility analysis). The results passed the futility analysis as seronegative patients treated with the antibody cocktail had a lower risk of death or receiving mechanical ventilation.
Regeneron currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Bristol Myers Provides Pipeline and Regulatory Updates: Bristol Myers BMY announced that the phase III study, CheckMate -548, evaluating the addition of immuno-oncology drug Opdivo (nivolumab) to the current standard of care (temozolomide and radiation therapy) versus placebo plus the standard of care in patients with newly diagnosed glioblastoma multiforme (GBM) failed. Following a routine review of the study by an independent data monitoring committee (DMC), the company was informed that based on the number of events to date, the study will not meet its primary endpoint of overall survival in patients with no baseline corticosteroid used or the overall randomized population. The DMC indicated that there were no safety concerns observed in patients treated with Opdivo that warranted stopping the study.
The company also announced that the European Medicines Agency (EMA) has validated its Marketing Authorization Application (MAA) for Zeposia (ozanimod) for the treatment of adults with moderately to severely active ulcerative colitis (UC).
Separately, the company also announced that it has decided to withdraw the indication of small cell lung cancer (SCLC) for Opdivo from the U.S. market, in consultation with the FDA. The drug was granted accelerated approval by the FDA in 2018 for the treatment of patients with SCLC whose disease has progressed after platinum-based chemotherapy and at least one other line of therapy. The accelerated approval was based on Opdivo’s effect on surrogate endpoints from the phase I/II CheckMate -032 trial in patients with advanced or metastatic solid tumors, which showed encouraging response rates and duration of response with Opdivo in SCLC. However, subsequent confirmatory studies in different treatment settings, CheckMate -451 and CheckMate -331, did not meet their primary endpoints of overall survival.
Myovant Gains on Pfizer Collaboration: Shares of Myovant Sciences MYOV surged after it announced a collaboration with Pfizer PFE for the development and commercialization of relugolix — a once-daily, oral gonadotropin-releasing hormone (GnRH) receptor antagonist — in oncology and women’s health in the United States and Canada.
Per the terms of the agreement, both companies will jointly develop and commercialize Orgovyx (relugolix) in advanced prostate cancer and, if approved, relugolix combination tablet (relugolix 40 mg, estradiol 1.0 mg, and norethindrone acetate 0.5 mg) in women’s health in the United States and Canada. Myovant will remain responsible for regulatory interactions and drug supply and continue to lead clinical development for relugolix combination tablet. Myovant will receive up to $4.2 billion, including an upfront payment of $650 million, $200 million in potential regulatory milestones for the FDA approval of relugolix combination tablet in women’s health, and tiered sales milestones of up to $2.5 billion in net sales upon reaching certain thresholds for prostate cancer and also for the combined women’s health indications. Myovant will also receive $50 million and be entitled to receive double-digit royalties on sales, if Pfizer exercises the option to commercialize relugolix in oncology outside of the United States and Canada, excluding certain Asian countries.
Novavax Initiates Phase III COVID-19 Vaccine Study: Novavax, Inc. NVAX initiated PREVENT-19, a phase III study in the United States and Mexico, to evaluate the efficacy, safety and immunogenicity of NVX-CoV2373, its COVID-19 vaccine candidate. The study will evaluate the efficacy, safety and immunogenicity of NVX-CoV2373 in up to 30,000 people aged 18 years and older compared with placebo. The study’s primary endpoint is the prevention of PCR-confirmed, symptomatic COVID-19. The key secondary endpoint is the prevention of PCR-confirmed, symptomatic moderate or severe COVID-19. Both endpoints will be assessed at least seven days after the second study vaccination in volunteers who have not been previously infected with SARS-CoV-2.
Aprea Plummets on Study Failure: Aprea Therapeutics, Inc APRE plunged 78% after the late-stage study evaluating the safety and efficacy of lead candidate, eprenetapopt, combined with azacitidine (AZA) versus AZA alone in TP53 mutant myelodysplastic syndromes (MDS) failed to meet the primary endpoint. The phase III study enrolled 154 MDS patients, randomized 1:1 to either the eprenetapopt with AZA arm or the AZA alone arm. The study did not meet the predefined primary endpoint of complete remission (CR) rate. The analysis of the primary endpoint at this data cut demonstrated a higher CR rate in the experimental arm receiving eprenetapopt with AZA as compared to the control arm receiving AZA alone but did not reach statistical significance. In the intention-to-treat population of 154 patients, the CR rate in the eprenetapopt with AZA arm was 33.3% compared to 22.4% in the AZA alone arm.
Arcturus Tanks on Study Update: Shares of Arcturus Therapeutics Holdings Inc. ARCT tanked after it released new data on its vaccine candidate, ARCT-021, for COVID-19. While the phase I/II results indicate that ARCT-021 leads to a potent immune response to SARS-CoV-2, the level of antibodies generated weren’t impressive.
Meanwhile, the company also received approval from the Singapore Health Sciences Authority to proceed with a phase II clinical study of its vaccine candidate, ARCT-021.
Performance
Medical - Biomedical and Genetics Industry 5YR % Return
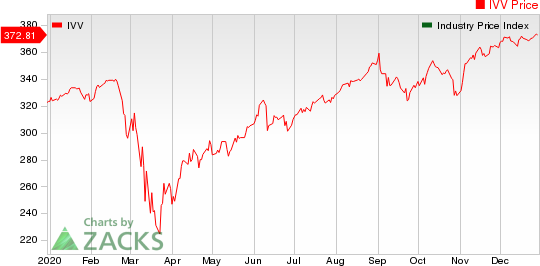
Medical - Biomedical and Genetics Industry 5YR % Return
The Nasdaq Biotechnology Index lost 2.16% in the last four trading sessions. Among the biotech giants, Amgen gained 2.66% during this period. Over the past six months, shares of Alexion have rallied 39.68%. (See the last biotech stock roundup here: Biotech Stock Roundup: PFE/BNTX Vaccine Authorized in Europe, Updates From AMGN, & More)
What's Next in Biotech?
Stay tuned for more pipeline and regulatory updates.
The Hottest Tech Mega-Trend of All
Last year, it generated $24 billion in global revenues. By 2020, it's predicted to blast through the roof to $77.6 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
Alcobra Ltd. (ARCT) : Free Stock Analysis Report
Myovant Sciences Ltd. (MYOV) : Free Stock Analysis Report
Aprea Therapeutics, Inc. (APRE) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
