Bristol-Myers' Opdivo Gets FDA Nod for Small Cell Lung Cancer
Bristol-Myers Squibb Company BMY announced that its immuno-oncology drug Opdivo obtained the FDA approval for the treatment of patients with metastatic small-cell lung cancer (SCLC), whose cancer has progressed after platinum-based chemotherapy and at least one other line of therapy.
The approval was based on positive overall response rate and duration of response from the SCLC cohort of the phase I/II CheckMate-032 trial.
The data from the study showed that out of 109 patients receiving Opdivo after platinum-based chemotherapy and at least one other prior line of therapy, 12% responded to the treatment based on the assessment by Blinded Independent Central Review (BICR), regardless of PD-L1 expression.
Per the company, Opdivo is now the first immuno-oncology treatment approved for small cell lung cancer (SCLC) in patients who received platinum-based chemotherapy and at least one other line of therapy.
SCLC accounts for 10% to 15% of all lung cancers. About 27,000 cases of SCLC are expected to be diagnosed in 2018 in the United States.
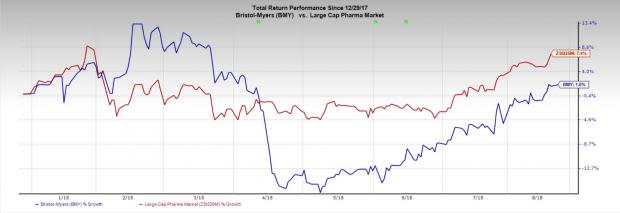
Bristol-Myers’ shares have gained 1.6% year to date compared with the industry’s gain of 7.4%.
Opdivo became the first PD-1 immune checkpoint inhibitor to gain regulatory approval in July 2014. It is currently approved in several countries including the United States, the EU and Japan for several cancer indications. Opdivo became the first PD-1 inhibitor to be approved for a hematological malignancy — classical Hodgkin lymphoma — in both the United States (May 2016) and the EU (November 2016).
In November 2016, Opdivo gained the FDA approval for the treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck (“SCCHN”) with disease progression on or after platinum-based therapy. The drug has been performing impressively due to demand resulting from the rapid commercial acceptance for several indications including melanoma, renal cell carcinoma and second-line non-small-cell lung cancer (“NSCLC”). The FDA also approved Opdivo for intravenous use in patients with hepatocellular carcinoma (HCC) and first-line RCC in the United States.
Label expansion into additional indications would give the product access to a higher patient population and increase the commercial potential of the drug significantly. Checkmate-227 met the co-primary endpoint of improved progression free survival for the Opdivo & Yervoy combination versus chemotherapy for the treatment of first-line NSCLC.
However, Opdivo faces stiff competition from Merck’s MRK Keytruda and Roche’s RHHBY Tecentriq.
Zacks Rank and Another Stock to Consider
Bristol-Myers currently carries a Zacks Rank #1 (Strong Buy). Another favourable stock in the healthcare sector is Gilead Sciences, Inc. GILD, which sports a Zacks Rank #1 as well. You can see the complete list of today’s Zacks #1 Rank stocks here.
Gilead’s earnings per share estimates have increased from $6.10 to $6.57 for 2018 in the last 60 days. Estimates for 2019 are also up by 14 cents.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot trades we're targeting>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research