Bristol Myers (BMY) Opdivo-Relatlimab Combo Gets Priority Review
Bristol Myers Squibb BMY recently announced that the FDA has accepted its biologics license application (BLA) for the fixed-dose combination of LAG-3-blocking antibody relatlimab and immuno-oncology Opdivo, administered as a single infusion, for the treatment of adult and pediatric patients (12 years and older and weighing at least 40 kg) with unresectable or metastatic melanoma.
The agency has granted Priority Review to the application and set a target action date of Mar 19, 2022.
The submitted BLA was based on positive efficacy and safety results of the phase II/III RELATIVITY-047 study, a phase II/III study evaluating the fixed-dose combination of relatlimab and Opdivo in patients with previously untreated metastatic or unresectable melanoma versus Opdivo alone. Data from the study showed that the fixed-dose combination of relatlimab and Opdivo achieved a statistically significant and clinically meaningful progression-free survival benefit over Opdivo monotherapy.
Melanoma is a form of skin cancer characterized by the uncontrolled growth of pigment-producing cells (melanocytes) located in the skin. Per the company, relatlimab is the first LAG-3-blocking antibody to demonstrate a clinical benefit for patients with phase III data.
The novel dual immunotherapy approach has the potential to improve outcomes for patients with metastatic or unresectable melanoma.
The successful development and commercialization of additional therapies will strengthen Bristol Myers’ oncology franchise.
Shares of the company have lost 2.4% year to date against the industry's decline of 1.3%.
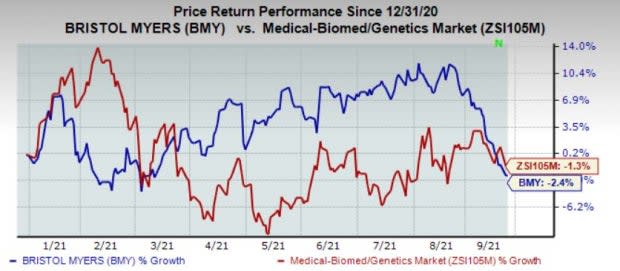
Image Source: Zacks Investment Research
Opdivo as monotherapy is currently approved for various indications — metastatic melanoma, metastatic non-small cell lung cancer (NSCLC), advanced renal cell carcinoma (RCC), among others. It is also approved for malignant pleural mesothelioma, NSCLC, and RCC in combination with Yervoy and for the first-line treatment of patients suffering from advanced RCC in combination with Exelixis’ EXEL cabozantinib.
Opdivo sales returned to growth in the second quarter after a lackluster first quarter. The approval of the drug either as monotherapy or in combination with other drugs for additional indications will boost its sales.
However, competition is very stiff for Opdivo for its various approved indications from Merck’s MRK Keytruda and Roche’s RHHBY Tecentriq.
Bristol Myers’ currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
