Bruker (BRKR) Assay to Offer Reliable Omicron Variant Detection
Bruker Corporation BRKR announced a major update related to its FluoroType SARS-CoV-2 varID Q real-time multiplexed polymerase chain reaction (PCR) assay. The company noted that this CE-IVD marked assay reliably detects all SARS-CoV-2 variants and is expected to provide a clear indication of Omicron (B.1.1.529). It is worth mentioning that the Omicron has been recently declared a ‘variant of concern’ by the World Health Organization (WHO).
More specifically, the FluoroType SARS-CoV-2 varID Q assay, based on Bruker´s proprietary LiquidArray technology, can detect and quantify all major SARS-CoV-2 variants. This assay simultaneously identifies the S-gene mutations Del69-70 and N501Y, which are anticipated to be clear indications of the novel Omicron (B.1.1.529) variant. The FluoroType SARS-CoV-2 varID Q also offers a viral load result according to WHO standard (IU/ml), CP values, and easy mutation interpretation.
Few Words on FluoroType SARS-CoV-2 varID Q
The FluoroType SARS-CoV-2 varID Q test contains reagents for 96 reactions and is verified for use with nasopharyngeal and oropharyngeal swabs. In less than 3-4 hours, sample extraction, amplification and PCR results are available. This assay can be run on the FluoroCycler XT PCR instrument after sample preparation with the GenoXtract fleXT system, which offers fully automated extraction and PCR setup. The FluoroSoftware analyzes results from the FluoroCycler XT, delivering easy-to-read results and direct indication of mutations.
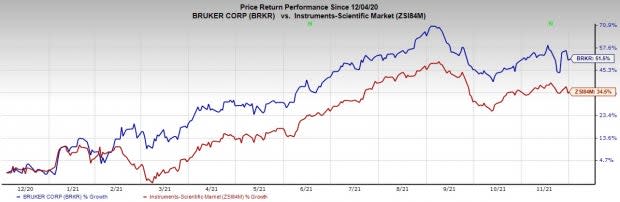
Image Source: Zacks Investment Research
The FluoroType SARS-CoV-2 varID Q assay enables users to run a screening tool for detecting SARS-CoV-2 viral load and get an early indication of the Omicron variant.
More on the News
Bruker also confirmed that the Omicron variant (B.1.1.529) is expected to be detected by their entire range of other FluoroType SARS-CoV-2 assays. These assays include CE-IVD assays such as FluoroType SARS-CoV-2 plus, FluoroType SARS-CoV-2 varID Q and FluoroType SARS-CoV-2/Flu/RSV. However, these assays are not available for sale in the United States. Another notable assay in this range involves the FluoroType SARS-CoV-2 evo Research Use Only (RUO), which is not available for use in clinical diagnostics procedures.
Bruker’s SARS-CoV-2 assays are CE-IVD-labeled per the European IVD Directive 98/79/EC and are primarily sold in European Markets. Notably, the FluoroType SARS-CoV-2 plus is registered and sold in various African markets, including South Africa.
Industry Prospects
Per a report by Fortune Business Insights published in GlobeNewswire, the global COVID-19 diagnostics market is expected to see a CAGR of 7.9% during 2020-2027. Factors such as the uncontrolled spread of coronavirus, and several efforts by medical and regulatory bodies to encourage innovation and accelerate research in developing coronavirus detection tools are expected to drive the market.
Given the market prospects, Bruker’s FluoroType SARS-CoV-2 varID Q assay, which detects all SARS-CoV-2 variants and expectedly the novel Omicron (B.1.1.529) variant, bears significant market potential.
Notable Developments
In November 2021, Bruker acquired MOLECUBES NV-- a dynamic innovator in benchtop preclinical nuclear molecular imaging (NMI) systems. The combination of Bruker's preclinical imaging technologies and global footprint with MOLECUBES' modular benchtop CUBES systems will offer a more extensive NMI offering, thereby accelerating preclinical NMI adoption at academic medical centers and biopharma firms worldwide.
In October 2021, Bruker secured orders for two 1.1 GHz NMR Avance Neo systems from National Science Foundation (NSF)-funded academic institutions in the United States. The Network for Advanced NMR (NAN) was established with funds from NSF and it connects three institutions, including the University of Connecticut School of Medicine, the University of Georgia and the University of Wisconsin–Madison.
Share Price Performance
The stock has outperformed its industry over the past year. It has grown 51.5% compared to the industry’s growth of 34.5%.
Zacks Rank and Other Key Picks
Currently, Bruker carries a Zacks Rank #2 (Buy).
Other top-ranked stocks in the broader medical space are Varex Imaging Corporation VREX, McKesson Corporation MCK and NextGen Healthcare, Inc. NXGN, each carrying a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Varex has a long-term earnings growth rate of 5%. The company surpassed earnings estimates in the trailing four quarters, delivering an average surprise of 115.3%.
Varex has outperformed the industry it belongs to in the past year. VREX has gained 73.7% versus the industry’s 8% fall.
McKesson has a long-term earnings growth rate of 8.9%. The company surpassed earnings estimates in the trailing four quarters, delivering a surprise of 19.9%, on average.
McKesson has outperformed its industry over the past year. MCK has gained 22% versus the 7.4% industry rise.
NextGen has a long-term earnings growth rate of 8.5%. The company surpassed earnings estimates in the trailing four quarters, delivering an average surprise of 16%.
NextGen has outperformed its industry over the past year. NXGN has declined 8.7% versus the industry’s 41.5% fall.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
McKesson Corporation (MCK) : Free Stock Analysis Report
Bruker Corporation (BRKR) : Free Stock Analysis Report
VAREX IMAGING (VREX) : Free Stock Analysis Report
NEXTGEN HEALTHCARE, INC (NXGN) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
