CARA Drug Wins FDA Nod for Pruritus Associated With CKD
Cara Therapeutics’ CARA lead product candidate Korsuva (CR845/difelikefalin) injection has been approved by the FDA for the treatment of moderate-to-severe pruritus associated with chronic kidney disease (CKD) in adults undergoing hemodialysis. The company’s shares surged significantly in response to the news.
The approval makes the drug the first and only therapy approved by the FDA for the treatment of pruritus associated with CKD in adults undergoing hemodialysis.
The new drug application (NDA) for Korsuva injection was granted Priority Review by the FDA.
Korsuva injection is a first-in-class kappa opioid receptor (KOR) agonist that targets the body’s peripheral nervous system.
The approval was supported by positive data from two pivotal late-stage studies — KALM-1, conducted in the United States and the global KALM-2 — as well as supportive data from an additional 32 studies.
Korsuva injection has demonstrated statistically significant reductions in itch intensity and concomitant improvement in quality of life measures in hemodialysis patients with moderate-to-severe CKD-associated pruritus (CKD-aP) in two phase III studies.
Cara has a license agreement with Vifor Pharma for the commercialization of the drug. Per the deal, the companies will share profits and losses received in connection with the commercialization of Korsuva in the United States. While Cara will get 60% of the profits, Vifor will receive 40% in non-Fresenius Medical Care clinics in the United States.
Under a previous agreement, Vifor Fresenius Medical Care Renal Pharma and Cara agreed to market Korsruva injection to Fresenius Medical Care North America dialysis clinics in the United States. Per this agreement, Cara would get 50% of the profit while Vifor Pharma will get the other 50%.
Vifor Pharma expects to begin promoting Korsuva injection in the first quarter of next year with reimbursement expected in the first half of 2022.
Cara’s stock has declined 5.7% in the year so far compared with the industry’s decrease of 2%.
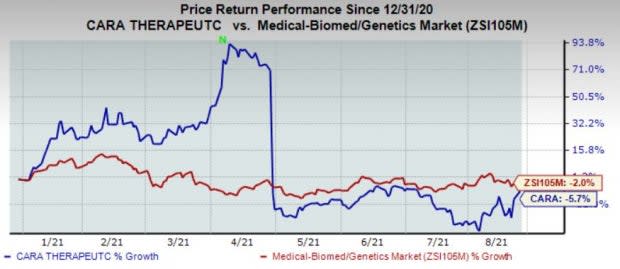
Image Source: Zacks Investment Research
The company is also developing an oral formulation for the treatment of pruritus in patients with CKD and atopic dermatitis and is currently in phase II studies in primary biliary cholangitis PBC and notalgia paresthetica (NP) patients with moderate-to-severe pruritus.
Zacks Rank & Stocks to Consider
Cara currently carries a Zacks Rank #3 (Hold). A few better-ranked stocks in the biotech sector are Horizon HZNP, Regeneron REGN and Repligen Corporation RGEN. All of them sport a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Horizon’s earnings per share estimates for 2021 have increased from $3.62 to $4.46 in the past 30 days. The stock has rallied 48.9% in the year so far.
Regeneron’s earnings per share estimates for 2021 have increased from $49.96 to $54.15 in the past 30 days. The stock has rallied 37.9% in the year so far.
Earnings estimates for Repligen for 2021 are up 50 cents in the past 30 days. The stock is up 39.4% in the year so far.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Horizon Therapeutics Public Limited Company (HZNP) : Free Stock Analysis Report
Repligen Corporation (RGEN) : Free Stock Analysis Report
Cara Therapeutics, Inc. (CARA) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
