Cassava (SAVA) Soars on Encouraging Alzheimer's Study Data
Cassava Sciences, Inc. SAVA announced encouraging interim data from an open-label study of its lead pipeline candidate, simufilam, for Alzheimer’s disease (“AD”). Data from the study showed that treatment for six months with the candidate led to improvement in patients’ cognition and behavior scores without any safety issues.
Data from this study along with previously completed data will serve as a basis for the phase III study on the candidate, scheduled to start in the second half of 2021.
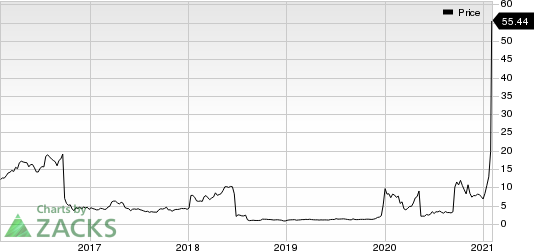
Shares of Cassava soared 141.2% on Feb 2 as investors were encouraged by the positive results of simufilam in the difficult-to-treat disease. In fact, the company’ shares have gained 563.9% in the past year compared with the industry’ increase of 2.9%.

The open-label study evaluated long-term safety and tolerability of twice-daily 100 mg dose of simufilam in patients with mild-to-moderate Alzheimer’s disease. Data from the analysis showed that cognition scores improved by 1.6 points or 10% (mean) from baseline as measured on ADAS-Cog11 scale, a standard test for assessing changes in cognition. Moreover, the candidate improved dementia-related behavior in AD patients by 29% (mean) or 1.3 points on the Neuropsychiatric Inventory from baseline, a widely used tool for this measurement. Based on the encouraging data, the company will increase the enrollment target to 150.
Meanwhile, the company has successfully completed an end-of-phase II meeting with the FDA whose details will be shared later in this quarter.
Please note that the company had announced encouraging final results from a phase IIb study evaluating sumifilam in patients with mild-to-moderate AD in September 2020 that suggest sumifilam may be slowing disease progression in Alzheimer’s patients. Additionally, AD patients treated with sumifilam showed directional improvements in tests of remembering new information compared to patients on placebo.
The company believes that the candidate has potential to provide lasting treatment effects for AD patients. The candidate has strong potential as there are no approved drugs for treating AD.
Although several other players are developing treatment for this disease, there is no success yet. Last month, Biohaven BHVN announced that its AD candidate, troriluzole, failed to achieve statistical improvement on ADAS-Cog11 scale. Biogen BIIB is one of the leading players in the AD segment with multiple candidates for treating AD. Companies that have terminated their AD programs following years of failure include Pfizer PFE and Merck.
Cassava Sciences, Inc. Price
Cassava Sciences, Inc. price | Cassava Sciences, Inc. Quote
Zacks Rank
Cassava currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
These Stocks Are Poised to Soar Past the Pandemic
The COVID-19 outbreak has shifted consumer behavior dramatically, and a handful of high-tech companies have stepped up to keep America running. Right now, investors in these companies have a shot at serious profits. For example, Zoom jumped 108.5% in less than 4 months while most other stocks were sinking.
Our research shows that 5 cutting-edge stocks could skyrocket from the exponential increase in demand for “stay at home” technologies. This could be one of the biggest buying opportunities of this decade, especially for those who get in early.
See the 5 high-tech stocks now>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Biohaven Pharmaceutical Holding Company Ltd. (BHVN) : Free Stock Analysis Report
Cassava Sciences, Inc. (SAVA) : Free Stock Analysis Report
To read this article on Zacks.com click here.
