Clovis Gains Rights to Pre-Clinical Cancer Program for $12M
Clovis Oncology, Inc. CLVS announced that it has entered into a global licensing and collaboration agreement with privately-held, Germany-based biotech 3B Pharmaceuticals GmbH. The deal grants Clovis U.S. and global rights (excluding Europe) to fibroblast activation protein alpha (FAP)-targeted radiopharmaceutical program. 3B Pharmaceuticals retains rights in Europe related to the FAP program. Please note that FAP is highly expressed in several epithelial cancers, including more than 90% of breast, lung, colorectal and pancreatic carcinomas..
Under the collaboration agreement, the initial focus will be on developing a peptide-targeted radionuclide therapy (PTRT) and imaging agent targeting FAP. Clovis will conduct global clinical studies and will solely bear the expenses during pre-clinical stage of development. Clovis expects to file an Investigational New Drug (“IND”) application for FAP-targeted radiopharmaceutical therapy in the second half of 2020.
Per the terms of the deal, Clovis will pay $12 million in upfront payment to 3B Pharmaceuticals. Clovis is also liable to pay certain development and regulatory milestone payments and commercial royalties on any potential future sales related to products developed under the program.
Meanwhile, Clovis and 3B Pharmaceuticals intend to collaborate on a discovery program for three additional targets for radionuclide therapy. 3B Pharmaceuticals will be responsible for discovery activities and Clovis will be responsible for IND-enabling studies.
So far this year, Clovis’ stock has declined 74.5% against the industry’s increase of 0.6%.

Please note that Clovis has one marketed drug — Rubraca — in its portfolio. This PARP inhibitor is approved as a maintenance treatment for recurrent ovarian cancer, irrespective of BRCA-mutation, in second-line setting. The drug is also approved as third or later-line treatment for BRCA-mutant ovarian cancer. Clovis is focused on expanding Rubraca’s label into other cancer indications.
The company recorded revenues of $66.1 million in the first six months of 2019, solely from sales of Rubraca, registering growth of nearly 56% year over year.
However, the company faces stiff competition from large-cap pharma companies including AstraZeneca AZN, Merck MRK and Glaxo GSK, which also market PARP inhibitors for ovarian cancer and are developing them for breast, prostate and other cancer indications. The diversification of Clovis’ pipeline through the acquisition of FAP program may help the company to fight competition in the long run.
Clovis Oncology, Inc. Price
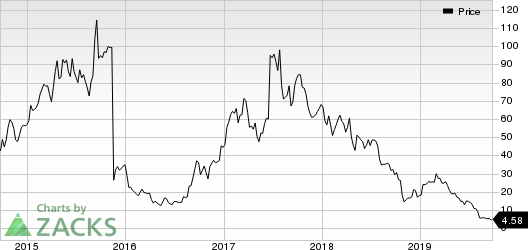
Clovis Oncology, Inc. price | Clovis Oncology, Inc. Quote
Zacks Rank
Clovis currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1%, and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Clovis Oncology, Inc. (CLVS) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
