Coherus (CHRS), Junshi Announce Positive NSCLC Study Results
Coherus BioSciences, Inc CHRS and partner Shanghai Junshi Biosciences Co., Ltd. announced positive interim results from a late-stage study evaluating toripalimab plus chemotherapy as first-line treatment of advanced squamous or non-squamous non-small cell lung cancer (NSCLC).
Toripalimab is an anti-PD-1 monoclonal antibody. The randomized, double-blind, placebo-controlled phase III study, “CHOICE-01”, evaluated toripalimab plus chemotherapy arm vis-à-vis placebo plus chemotherapy arm.
A total of 465 treatment-naive advanced NSCLC patients (220 squamous and 245 non-squamous) were randomized in the ratio of 2:1.
The interim results showed that toripalimab plus chemotherapy met primary endpoint with significant improvement in progression free survival (PFS) compared to chemotherapy alone. The 1-year PFS rates for toripalimab and placebo arms were 32.6% and 13.1%, respectively. This improvement in PFS was observed in both squamous and non-squamous NSCLC and regardless of PD-L1 expression.
Data support the use of toripalimab with chemotherapy as first-line therapy for patients with NSCLC. Toripalimab in combination with chemotherapy, compared with chemotherapy alone, resulted in better objective response rate (ORR) and median duration of response (DoR).
Overall survival data were not yet mature as of Mar 7, 2021.
The candidate is also being evaluated in late-stage studies in esophageal, lung, liver, breast, kidney, bladder, stomach, and skin cancers.
Shares of Coherus have declined 12% in the year so far compared with the industry’s 0.2% decline.
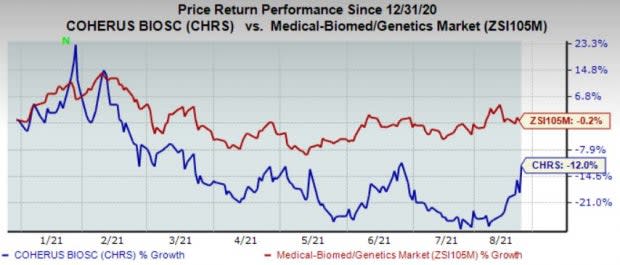
Image Source: Zacks Investment Research
A rolling submission of the first toripalimab Biologics License Application (BLA) is underway in the United States for the treatment of recurrent or metastatic nasopharyngeal carcinoma (NPC). The FDA has granted Breakthrough Therapy designation to toripalimab in combination with chemotherapy for the first-line treatment of recurrent or metastatic NPC and also for toripalimab monotherapy in second or third line treatment of recurrent or metastatic NPC.
Toripalimab was the first domestic anti-PD-1 monoclonal antibody approved in China as Tuoyi.
While the NSCLC markets represent potential, competition is stiff from bigwigs like Merck’s MRK Keytruda, Roche’s RHHBY Tecentriq and Bristol Myers’ BMY Opdivo.
Coherus currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Coherus BioSciences, Inc. (CHRS) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
