CVM: Data Has Been Released
NYSE:CVM
READ THE FULL CVM RESEARCH REPORT
Data Readout From the IT MATTERS Trial
On June 28, 2021, CEL-SCI Corporation (NYSE:CVM) reported selected data from the IT-MATTERS trial. While details for the primary endpoint were not provided, an important subset of the population in the Multikine arm produced a statistically significant 14.1% improvement in overall survival (OS) relative to standard of care (SoC). The p-value for the 5-year result was 0.0236 and the Hazard Ratio (HR) was 0.681 in a population representing about 40% of all advanced primary squamous cell carcinoma of the head and neck (SCCHN) patients. We summarize below the key data provided by the company:
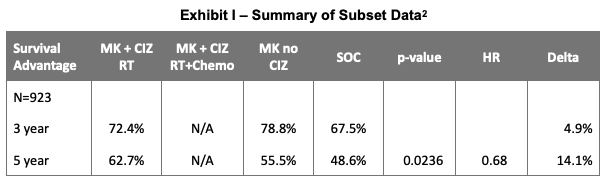
Additional detail provided by the company in a discussion following the July 1st Annual Shareholder Meeting identified a total of 923 patients in the intent to treat (ITT) group which were broken down into three divisions: Low risk, which did not receive chemotherapy, high risk, which did receive chemotherapy and exclusions, which captured patients who had been randomized, received Multikine, but did not elect to move on to SoC. Group sizes were 380 (41.2%) for the low risk (no chemo) group, 467 (50.6%) for the high risk (administered chemo) group and 76 in the exclusion group.
The cancer patient population exhibiting the response in the trial was at the advanced (stage III and IV) primary (not yet treated) SCCHN. Safety for the trial population who were treated with Multikine, did not raise any safety concerns and matched the favorable results demonstrated earlier trials. No safety issues were found related to drug administration with no detection of late adverse effects.
Few details were released regarding the primary endpoint except that the press release noted that the study did not achieve the 10% improvement in OS in the combined study groups. We anticipate that this information will be made available later.
CEL-SCI, while blinded to the study, developed prospective several statistical analyses for the population prior to data lock which examined multiple groups likely to benefit from Multikine. The company believes that the early identification of the Multikine neoadjuvant population exhibiting a 14.1% OS advantage at five years will be amenable to the FDA as it reviews the data. In an area of unmet need, such as head and neck cancer, the bar is lower for determined endpoints and we anticipate a near-term meeting with the FDA will provide additional clarity. Safety is a strong point with Multikine, which showed no safety issues in the Phase III trial nor in previous studies compared with SoC and with other immunotherapies that are associated with cytokine storm and other negative side effects. We think it is likely that the agency will look favorably upon a new treatment for an unmet need that is safe. To this point, we highlight the case of aducanumab, which demonstrated minimal, if any, efficacy, but was approved by the FDA given the substantial unmet need. We discuss the FDA’s thinking on this matter in a recent article here which may be applicable to CEL-SCI’s situation as well.
Now that the company has made its announcement, key drivers for valuation include the FDA’s willingness to accept the data available for a Biologic License Application (BLA) consideration. Additional data and information may be required prior to acceptance. The company has reached out to the FDA and contacted representatives from both the offices of oncology products and rare disease. We will update investors on these meetings, their outcomes and impact on valuation when details are made available.
Multikine Near Term Milestones
➢ Release of subset data for IT MATTERS – June 28, 2021
➢ Development of clinical study report – 2021
➢ Request meeting with FDA to determine path forward – 2021
➢ Development of paper for publication in peer reviewed journal - 2021
➢ Presentation of data package to review with FDA – 2021
➢ Address FDA Comments – 2021/2022
➢ Submission of BLA to FDA – 2022
Letter to Shareholders
On July 7, 2021, CEL-SCI CEO, Geert Kersten, issued a Letter to Shareholders. The purpose of the letter was to clarify whether or not the data recently shared with the public can be used in a BLA for submission to the FDA. Several points were made, including that the successful treatment arm was pre-specified in the protocol and conducted before unblinding, the five year data generated exceeded the 10% threshold and was statistically significant. The press release further noted that, if successfully approved, Multikine would be appropriate for 155,000 SCCHN patients per year, and that it would be addressing an unmet need in a serious disease. We reiterate the company’s argument for a successful submission:
➢ Primary efficacy target met in pre-specified population
➢ Statistical significance reached
➢ Model results are robust and extend to five years
➢ No safety issues
➢ Multikine is a product that has demonstrated efficacy in a identifiable orphan population and an unmet need offering favorable reward to risk for patients
IT-MATTERS Trial Background
After almost a decade, the IT-MATTERS clinical trial experienced its final event, reported in a press release on May 4, 2020. The event-driven trial for head and neck cancer added its first patients in 2011 in the US, Canada, UK, France and 20 other countries. 928 patients were enrolled, with the final individual treated in September 2016. The primary endpoint for the study was an overall survival benefit of 10% over standard of care alone in defined areas of the head and neck.
The trial enrolled three arms including:
1) Multikine plus CIZ (M+CIZ) followed by SOC
2) Multikine (CIZ-exclusion) followed by SOC
3) SOC therapy as the active comparator
Only the M+CIZ and SOC groups will contribute to the event total for the trial. Over the duration of the trial, an independent data monitoring committee (IDMC) provided periodic checks approximately every 6 months to ensure patient interests were met and the trial was conducted ethically. In December 2020, the study entered its final stage of statistical analysis of all study data.
Valuation
Although we were only given limited information regarding the data from the IT-MATTERS trial, it was sufficient to refine our analysis and model and provide an updated valuation. We make three changes. The first is a reduction in the addressable population to reflect the 40% of patients that do not receive chemotherapy out of the advanced primary squamous cell head and neck carcinoma patients. We assume a similar penetration rate into this population that peaks at 40% of the, now modified, addressable market by the fifth year of commercialization. The second change is to increase the probability of success from 70% to 85% to reflect the statistically significant results generated by Multikine in the population treated by surgery and radiotherapy. The third modification is to push back our revenue estimates by approximately six months, with first sales occurring in 2023. The net result is a change of our target price to $12 per share.
CEL-SCI provided detail on the updated market size that are similar to the estimates we included in our initiation. The numbers below include the extra cut to identify the size of the low risk population that does not receive chemotherapy (only surgery followed by radiotherapy) based on market data provided by CEL-SCI.

Due to the limited information provided, the uncertainty regarding the FDA’s requirements following the pre-BLA meeting, the timeline over which a BLA will be submitted and the likely penetration rate given the stronger than expected benefit in a smaller population, our valuation will be in flux. We anticipate additional updates from the company regarding FDA interaction will help us refine the valuation in the months to come. Additional information regarding the magnitude and statistical significance of the primary endpoint will also be helpful in determining the need for additional data and the FDA’s willingness to accept the results.4
Summary
CEL-SCI reached the final event in the IT-MATTERS trial in May 2020 and released selected data for a key subpopulation in June 2021. While the company did not release information regarding the primary endpoint, it did demonstrate a meaningful advantage over SoC in the MK+Surgery+RT population. Meanwhile, CEL-SCI is making improvements to its manufacturing facility in anticipation of commercialization with completion expected soon. Based on management commentary, the facility has the ability to serve 12,000 to 13,000 patients per year and opportunity to expand on greater need.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.
________________________
1. The 0.68 Hazard Ratio was better than and below the pre-specified cutoff of 0.721
2. Compiled by Zacks’ analysts from company press release
3. Compiled by Zacks’ analysts from company provided data
4. SoC is taken directly from the NCCN guideline. With few alternatives, physicians will likely be favorably disposed to prescribing Multikine based on the lack of safety issues and the favorable 5-year OS.
