Emergent (EBS) Begins Dosing in Study on Influenza Vaccine
Emergent BioSolutions Inc. EBS announced that it has dosed the first participant in a phase I study evaluating its investigational universal influenza vaccine candidate, EBS-UFV-001. The study is being sponsored by Emergent and conducted in Australia.
The current version of this influenza vaccine candidate has multiple components intended to prompt broad and supra-seasonal immunity against influenza A viruses.
The double-blind, placebo-controlled dose-escalation study will evaluate the safety, tolerability, and immunogenicity of EBS-UFV-001 at two dose levels and two schedules in healthy adults aged between 18 to 45 years. The company’s universal influenza vaccine candidate is based on a nanoparticle vaccine.
Shares of Emergent have plunged 52.5% so far this year compared with the industry’s decline of 20.3%.
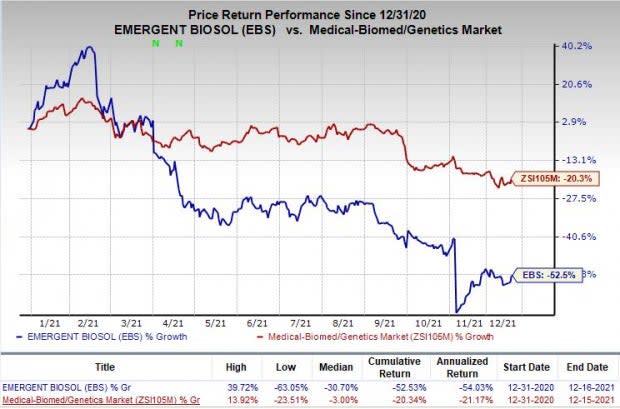
Image Source: Zacks Investment Research
Per the company, influenza A and B viruses cause seasonal epidemics of disease, which can lead to severe illness or death especially in a high-risk population. Hence, if successfully developed and upon potential approval, the influenza vaccine can offer protection to millions who are affected by the disease every year.
Please note that several other companies are engaged in developing influenza vaccine candidates, which can induce stiff competition to Emergent.
Moderna MRNA is evaluating mRNA-1010, its quadrivalent mRNA-based vaccine candidate, against seasonal influenza.
mRNA-1010 is Moderna’s first seasonal influenza vaccine candidate, which has entered clinical testing for the prevention of influenza, including seasonal influenza A H1N1, H3N2 and influenza B Yamagata and Victoria. Moderna will soon begin mid-stage studies on mRNA-1010.
Novavax NVAX is also developing its nanoparticle seasonal influenza vaccine candidate, NanoFlu, in late-stage studies. The company has completed a pivotal phase III study on NanoFlu for patients aged 65 years and above.
If approved, Novavax believes that NanoFlu will be a game-changer for the prevention of influenza.
Zacks Rank & Stock to Consider
Emergent currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the biotech sector is Sarepta Therapeutics, Inc. SRPT, which has a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Loss per share estimates for Sarepta Therapeutics have narrowed 28.2% for 2021 and 24.5% for 2022 over the past 60 days.
Earnings of Sarepta Therapeutics surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
Emergent Biosolutions Inc. (EBS) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
