Emergent (EBS) Gets Extra $23M for COVID-19 Vaccine Production
Emergent BioSolutions Inc. EBS announced that the U.S. Department of Health & Human Services’ Biomedical Advanced Research and Development Authority, or BARDA, has increased its task order by $23 million to support further expansion of manufacturing capacity for Johnson & Johnson’s JNJ COVID-19 vaccine.
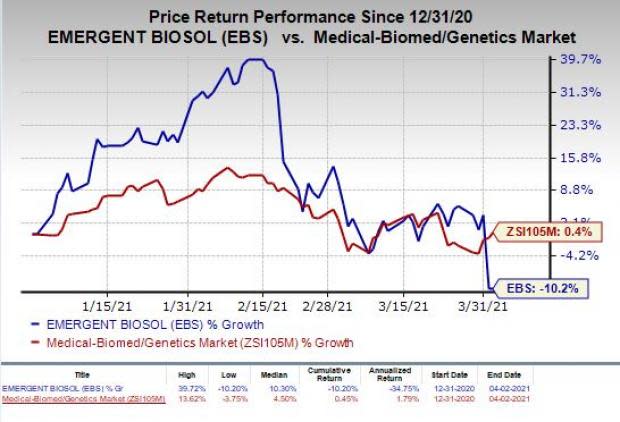
Shares of Emergent have declined 10.2% so far this year against the industry’s increase of 0.4%.
We remind investors that last week J&J and Emergent issued statements that a batch of drug substance for manufacturing the former’s COVID-19 vaccine at Emergent’s Bayview facility had failed to meet quality standards. A few weeks ago, reportedly, workers at Emergent's Bayview facility had mixed up ingredients of J&J’s and AstraZeneca’s AZN vaccines.
Emergent has partnered with both J&J and AstraZeneca to help manufacture their respective COVID-19 vaccines. The incident reportedly ruined 15 million doses of J&J’s vaccine. U.S. health officials have now put J&J in charge of the facility, implying that the plant will now only make J&J’s vaccine to rule out any future mix ups. The facility will cease production of AstraZeneca shots.
Despite the potential delay in shipments due to the Bayview blunder, J&J targets to deliver 24 million doses to the U.S. government in April and 100 million doses by the end of May.
Please note that AstraZeneca’s vaccine has not yet been granted EUA in the United States by the FDA.
In a separate press release, Emergent announced top-line data from the NIAID-sponsored phase III ITAC study (INSIGHT-013) evaluating four immunoglobulin candidates, including Emergent’s investigational SARS-CoV-2 Immune Globulin Intravenous (Human) (COVID-HIG), for the treatment of hospitalized patients with COVID-19.
The above-mentioned study investigated the safety and efficacy of four immunoglobulin candidates plus standard of care as compared to placebo plus standard of care in the given patient population.
Data from the study showed that the addition of anti-SARS-CoV-2 hyperimmunoglobulin to standard of care, inclusive of Gilead’s GILD Veklury (remdesivir), for hospitalized adult COVID-19 patients with symptoms for less than 12 days did not provide clinical benefit when compared to standard of care plus placebo.
Zacks Rank
Emergent currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Bitcoin, Like the Internet Itself, Could Change Everything
Blockchain and cryptocurrency has sparked one of the most exciting discussion topics of a generation. Some call it the “Internet of Money” and predict it could change the way money works forever. If true, it could do to banks what Netflix did to Blockbuster and Amazon did to Sears. Experts agree we’re still in the early stages of this technology, and as it grows, it will create several investing opportunities.
Zacks’ has just revealed 3 companies that can help investors capitalize on the explosive profit potential of Bitcoin and the other cryptocurrencies with significantly less volatility than buying them directly.
See 3 crypto-related stocks now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
