Exelixis (EXEL), Sairopa Obtains IND Clearance for ADU-1805
Exelixis, Inc. EXEL announced that the FDA has cleared partner Sairopa B.V.’s investigational new drug (IND) application to evaluate the safety and pharmacokinetics of ADU-1805 in adults with advanced solid tumors.
Both companies entered into an exclusive clinical development and option agreement for ADU-1805, a potentially best-in-class monoclonal antibody that targets SIRPα, in November 2022.
Exelixis has the option to obtain an exclusive, worldwide license to develop and commercialize ADU-1805 and other anti-SIRPα antibodies upon review of data from prespecified phase I studies of ADU-1805 to be completed by Sairopa during the option period. The IND clearance triggers a $35 million milestone payment from Exelixis to Sairopa. The payment will be made in the first quarter of 2023.
A phase I study of ADU-1805 as a single agent and in a combination regimen in advanced solid tumors is expected to begin in the second quarter of 2023.
Exelixis’ lead drug, Cabometyx (cabozantinib), is approved for advanced renal cell carcinoma (RCC) and previously treated hepatocellular carcinoma (HCC). The company is making efforts to develop its portfolio beyond Cabometyx.
It also entered into an exclusive collaboration agreement with Cybrexa Therapeutics in November 2022, which gave it the right to acquire CBX-12 (alphalex exatecan), a clinical-stage, first-in-class peptide-drug conjugate (PDC) that utilizes Cybrexa’s proprietary alphalex technology to enhance the delivery of exatecan to tumor cells.
Exelixis’ shares have lost 4.2% in the past year compared with the industry’s decline of 7.6%.
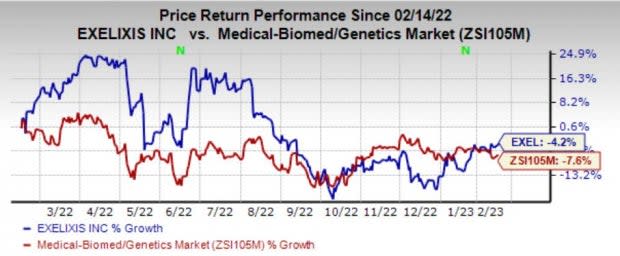
Image Source: Zacks Investment Research
Concurrently, Exelixis and partner Bristol-Myers BMY announced encouraging three-year follow-up results from the phase III CheckMate-9ER study. The results demonstrated sustained survival and response rate benefits with the combination of Opdivo (nivolumab) and Cabometyx versus sunitinib in the first-line treatment of advanced RCC. In addition, a biomarker analysis showed that improvements in median progression-free survival (PFS) and overall survival (OS) were sustained with the combination of Opdivo and Cabometyx, regardless of PD-L1 status.
Please note that the FDA approved Cabometyx in combination with Opdivo in 2021 as a first-line treatment for patients with advanced RCC.
Bristol-Myers’ Opdivo, one of its leading revenue generators, is approved for various oncology indications.
Exelixis’ recently released fourth-quarter results were decent. Demand continues to grow based on sales volume being driven by the Cabometyx and Opdivo combination.
Zacks Rank & Stocks to Consider
Exelixis currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the biotech sector are Alkermes ALKS and Dynavax Technologies DVAX, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Over the past 60 days, earnings estimates for Alkermes for 2022 have increased by 2 cents to 25 cents. Alkermes surpassed estimates in three of the trailing four quarters and met the same in the remaining one, the average surprise being 306.73%.
Over the past 60 days, earnings estimates for Dynavax for 2022 have increased by 11 cents to $1.95. Dynavax surpassed estimates in two of the trailing four quarters and missed in the other two, the average surprise being 73.15%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Alkermes plc (ALKS) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
