Four more hand sanitizers recalled as ‘toxic.’ The FDA’s ‘Do Not Use’ list is up to 165
Four more hand sanitizers on the FDA’s Do Not Use list have been recalled, one of them among the additions over the past week that pushed that list count to 165.
Most hand sanitizers are on the list because they contain methanol, also known as wood alcohol. As explained by Asiaticon SA de CV in its company-written, FDA-posted recall notice about the products below:
“Methanol has inferior antiseptic properties compared to ethanol. Substantial methanol exposure can result in nausea, vomiting, headache, blurred vision, permanent blindness, seizures, coma, permanent damage to the nervous system or death.
“Although all persons using these products on their hands are at risk, young children who accidentally ingest these products and adolescents and adults who drink these products as an alcohol (ethanol) substitute, are most at risk for methanol poisoning.”
The recalls
▪ Citing possible methanol presence and weak ethanol content, Asiaticon pulled all of its V-Klean Hand Sanitizer in 8.5-ounce, 16.9-ounce and 33.8-ounce bottles , Medically Minded Hand Sanitizer in 16.9-ounce bottles and Protz Real Protection Antibacterial, NDC No. 75192-600-02, hand sanitizers in 13.5-ounce bottles.

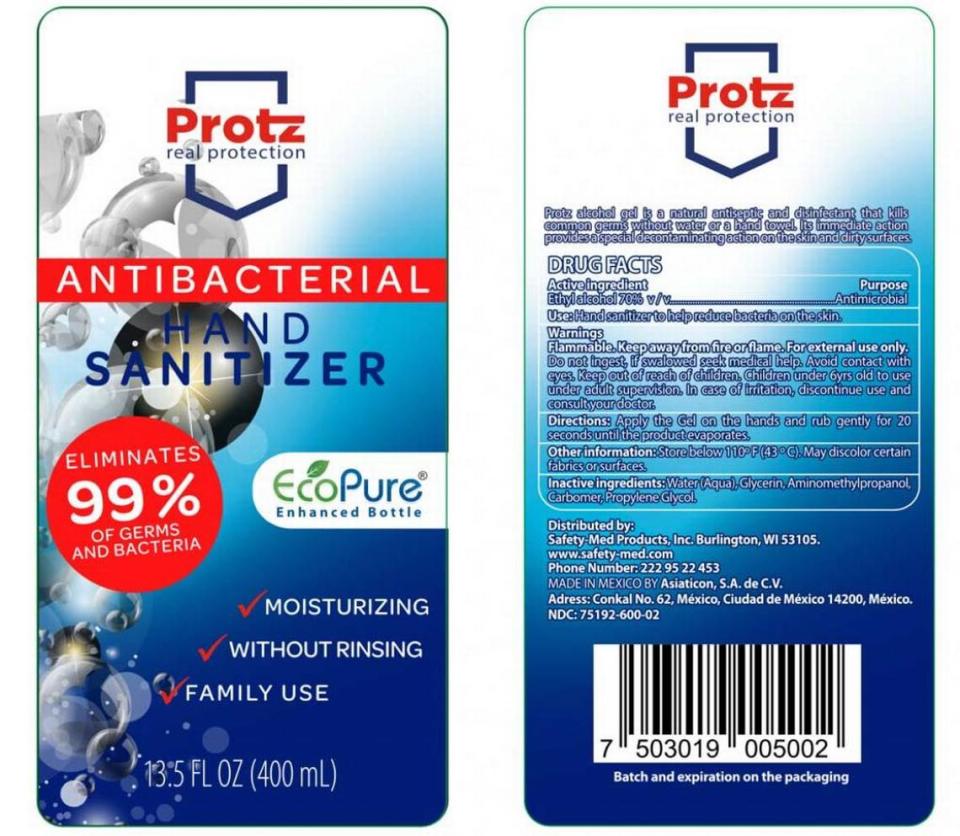

These can be returned to the place of purchase for a full refund. Those with questions should call 929-394-3020, Monday through Friday, 9:30 a.m. to 6 p.m., Eastern time.
▪ Nanomateriales recalled all lots of Zanilast + Gel Sanitizer, 1-liter, 25-liter and 1-gallon sizes, which went to California, New York and New Jersey between May 29 and June 17.

Customers with questions about this recall can call 210-963-5679, Monday through Friday, 11 a.m. to 6 p.m., Eastern time, or e-mail contacto@nanomateriales.com.mx.
Anyone having medical problems from using any of these products should talk to a medical professional. Then report it to the FDA either at their Adverse Event reporting site or by requesting a printed form at 800-332-1088.
Updates to the ‘Do Not Use’ list
As stated above, methanol presence filled most of the list. But having a microbial contamination, 1-propanol (also called “toxic”), a subpotent active ingredient or being made in the same facility as a hand sanitizer with any of the above problems also gets a listing.
▪ DMM Vission’s SYP Health Hand Sanitizer Alcohol Gel, National Drug Code Nos. 75799-000-01, 75799-000-02, 75799-000-03, 75799-000-04, tested as having methanol.

▪ Jose Miguel Gutierrez Salas’ MVP Sanitizing Services Spray Hand Sanitizer tested as having methanol. Though it has a label on the NDC site, the FDA says it has “no evidence this product is in the U.S. market.”
▪ MYM Hidrominerales’ version of Protz Real Protection Antibacterial Hand Sanitizer, NDC No. 77872-600-40, tested as having methanol.
▪ As stated above, Nanomateriales’ Zanilast + Gel Sanitizer has been recalled. The Zanilast distributed by Nanomateriales tested as having 1-propanol. The Zanilast made by Nano, but distributed by Fujimura Trading made the Do Not Use list by association.
Here’s a new peach salmonella update that includes Costco recalls, Walmart’s store list
Two Florida wings restaurants didn’t pay workers $115,000 after closing for COVID-19
