Gilead (GILD) Breast Cancer Drug Trodelvy Improves Survival
Gilead Sciences, Inc. GILD has announced positive data from the phase III TROPiCS-02 study on breast cancer drug Trodelvy (sacituzumab govitecan-hziy) on the key secondary point of overall survival (OS).
Trodelvy is a first-in-class Trop-2-directed antibody-drug conjugate.
The study is evaluating Trodelvy vis-à-vis comparator chemotherapy (physicians’ choice of chemotherapy, TPC) in patients with HR+/HER2- metastatic breast cancer who received endocrine-based therapies and at least two chemotherapies.
Results showed Trodelvy demonstrated a statistically significant and clinically meaningful improvement of 3.2 months in OS compared to TPC. Gilead will present the data at the European Society for Medical Oncology (ESMO) Congress 2022.
Other key secondary endpoints, including objective response rate (ORR), demonstrated statistically significant improvement in favoring Trodelvy versus TPC. Time to deterioration (TTD) of Global Health Status/Quality of Life (QoL) and Fatigue scale per EORTC-QLQ-C30 also favored Trodelvy versus TPC. No statistically significant difference in TTD on the Pain Scale was observed.
The survival benefit of over three months for patients with pre-treated HR+/HER2- metastatic breast cancer highlight the potential for sacituzumab govitecan in patients with pre-treated HR+/HER2- metastatic breast cancer.
Trodelvy is approved in multiple countries for treating adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Trodelvy has now demonstrated a survival benefit in both pre-treated HR+/HER2- metastatic breast cancer and second-line metastatic TNBC – two difficult-to-treat forms of breast cancer. Gilead has submitted a supplemental biologics license application (sBLA) to the FDA based on data from TROPiCS-02.
The drug is also approved in the United States under the accelerated approval pathway for the treatment of adult patients with locally advanced or metastatic urothelial cancer (UC) who have previously received platinum-containing chemotherapy and either programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor.
Earlier in the week, Gilead also announced positive new data from a post hoc subgroup analysis from the phase III TROPiCS-02 study. The analysis examined progression-free survival (PFS) in the intention-to-treat population by HER2-immunohistochemistry (IHC) status. Results showed Trodelvy’s efficacy across HER2-low and IHC0 status in pre-treated metastatic breast cancer patients. Trodelvy improved median PFS versus TPC in both HER2-low (IHC1+ and IHC2+/ISH-negative) and IHC0 groups.
Gilead’s stock has lost 10.2% so far in the year compared with the industry's decline of 24%.
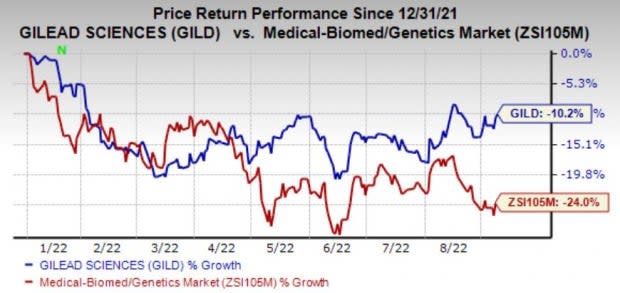
Image Source: Zacks Investment Research
With Trodelvy and its CAR T franchise, Gilead is making efforts to develop its oncology business to diversify its revenue base.
Trodelvy is also being developed for potential investigational use in other TNBC and metastatic UC populations, as well as a range of tumor types where Trop-2 is highly expressed, including metastatic non-small cell lung cancer (NSCLC), metastatic small cell lung cancer (SCLC), head and neck cancer, and endometrial cancer.
While the HIV business maintains momentum on the back of Biktarvy and approval of new treatments, competition is stiff in the HIV business from the likes of GSK plc GSK.
GSK’s HIV franchise recorded 7% growth in the second quarter. Growth was driven by new HIV products like Dovato, Cabenuva, Rukobia, Juluca and Apretude, and a favorable U.S. pricing mix.
Gilead currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the sector are Bolt Pharmaceuticals BOLT and Dynavax DVAX . Both carry a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Loss estimates for BOLT have narrowed to $2.54 from $2.87 in the past 60 days. BOLT surpassed earnings in three of the trailing four quarters, the average being 2.39%.
Dynavax’s earnings estimates have increased to $1.73 from $1.14 for 2022 over the past 60 days. Earnings of Dynavax surpassed estimates in two of the trailing four quarters.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Bolt Biotherapeutics, Inc. (BOLT) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
