Glaxo (GSK)/Sanofi Delay Study on Coronavirus Vaccine Candidate
GlaxoSmithKline plc GSK and Sanofi SNY announced that they have decided to delay the development plan of their adjuvanted recombinant protein-based COVID-19 vaccine candidate, which is currently being evaluated in a phase I/II study. The companies took the decision due to insufficient immune response observed in older adults in early-stage studies.
Glaxo’s pandemic adjuvant technology is being combined with Sanofi’s recombinant protein-based technology to develop the adjuvanted COVID-19 vaccine.
Interim data from a phase I/II study showed that in adults aged 18 to 49 years, the candidate showed an immune response comparable to patients who recovered from COVID-19. However, in older adults, the vaccine candidate demonstrated a low immune response, which may be due to an insufficient concentration of the antigen. The phase I/II clinical study on the candidate was initiated in September.
The companies now plan to begin the phase IIb study with an improved antigen formulation in February next year. The study will involve a comparison with an authorized COVID-19 vaccine. If data from this study is found to be positive, Glaxo/Sanofi will start the phase III study in the second quarter of 2021.
The abovementioned study will be supported by the Biomedical Advanced Research and Development Authority as a part of the U.S. government’s Operation Warp Speed.
Regulatory filing for the COVID-19 vaccine candidate is now expected in the second half of 2021, delayed from the earlier expectation of regulatory filings in the first half. If everything goes well, the vaccine is expected to hit the market in the fourth quarter of 2021, delayed from the earlier projection of mid-2021.
We note that Glaxo/Sanofi have already signed supply agreements with the European Union, the United States government and other countries for delivering the vaccine candidate upon potential approval. Hence, this delay does not bode well for the companies in the long run.
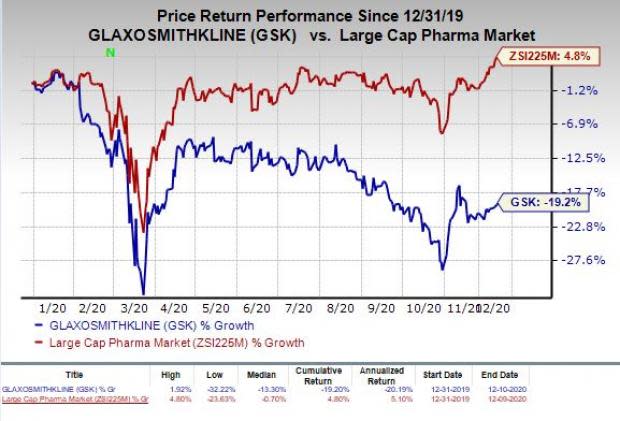
Shares of Glaxo have plunged 19.2% so far this year against the industry’s increase of 4.8%.
Glaxo has formed several similar collaborations to make its pandemic adjuvant technology available to partners who are making adjuvanted COVID -19 vaccine candidates. In November 2020, Glaxo and partner Medicago started a phase II/III study of their adjuvanted COVID-19 vaccine candidate.
We note that apart from Glaxo and Sanofi, several pharma/biotech companies are making painstaking efforts to make a COVID-19 vaccine available for curing the pandemic.
As of now, Pfizer PFE and partner BioNTech’s BNTX COVID-19 vaccine candidate, BNT162b2, is the only vaccine which has been approved for emergency use in the United Kingdom, Bahrain and Canada. Moreover, the companies have received a favorable vote from an FDA panel recommending approval for emergency use authorization to BNT162b2.
Zacks Rank
Glaxo currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by referendums and legislation, this industry is expected to blast from an already robust $17.7 billion in 2019 to a staggering $73.6 billion by 2027. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot stocks we're targeting >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
