GSK's RSV Vaccine Candidate Offers Robust Protection in Elderly
GSK plc GSK announced positive headline data from a pre-specified efficacy interim analysis of the phase III study — AReSVi 006 — evaluating its respiratory syncytial virus (RSV) vaccine candidate for adults aged 60 years and above or older adults or elderly.
Data from the interim analysis showed that the vaccine candidate achieved statistically significant and clinically meaningful efficacy. The strong efficacy was achieved against RSV A and B strains as well as in individuals more elderly individuals — aged 70 years and above.
Data from the late-stage study was reviewed by an Independent Data Monitoring Committee, which observed that the study’s primary endpoint was exceeded with no unexpected safety concerns.
GSK’s chief scientific officer claimed that data from the study showed the potential of its RSV vaccine candidate in offering exceptional protection for older adults from the serious consequences of RSV infection.
Currently, there are no FDA-approved vaccines against RSV infections — a disease that leads to 360,000 hospitalizations and more than 24,000 deaths worldwide each year. GSK is planning to engage with regulatory bodies in different countries immediately to discuss the potential approval pathway. It is planning to file regulatory applications seeking approval for its RSV vaccine candidate for older adults in the second half of 2022.
The AReSVi 006 study will continue to evaluate the RSV vaccine candidate for an annual revaccination schedule as well as evaluation of longer-term protection over multiple seasons following one dose in older adults.
Shares of GSK have lost 0.7% so far this year against the industry’s increase of 1%.
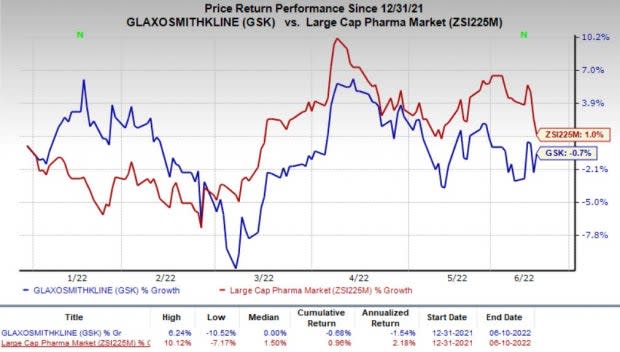
Image Source: Zacks Investment Research
GSK boasts a strong portfolio of vaccines approved for various indications. In the first quarter of 2022, vaccine sales gained 36% at constant exchange rate, mainly driven by strong demand recovery in Shingrix (shingles vaccines) sales. In 2022, GSK expects vaccine sales to grow at low-teens percentage points, excluding pandemic solutions.
An approval from the FDA for Priorix earlier this month is likely to bring additional sales for GSK’s vaccine portfolio. Priorix is approved for active immunization for the prevention of measles, mumps and rubella in individuals 12 months of age and older. GSK offered to acquire privately-held biopharmaceutical company, Affinivax, last month to boost its already strong vaccines portfolio. The acquisition, which is set to be completed in the third quarter of 2022, will add a new, potentially disruptive technology to build a strong portfolio of innovative vaccines as well as specialty medicines.
Meanwhile, a potential approval for the RSV vaccine for older adults will boost GSK’s vaccine portfolio as it may become the first FDA-approved vaccine for this patient population.
Please note that GSK is spinning-off its Consumer Healthcare business into a separate public unit, Haleon, to focus more on its Specialty Medicines and Vaccines portfolio. The Consumer Healthcare business is a joint venture between GSK and Pfizer PFE, which hold 68% and 32% stakes, respectively. The demerger will be completed next month.
Following the demerger, GSK’s shareholders will hold at least 54.5% of Haleon and GSK itself will hold up to 6%. An aggregate of 7.5% of Haleon will be held by certain Scottish limited partnerships, set up to provide additional funding for GSK’s UK Pension Schemes. Pfizer is expected to maintain its ownership stake of 32% in Haleon. GSK and Pfizer plan to exit their ownership stakes in Haleon.
GlaxoSmithKline plc Price
GlaxoSmithKline plc price | GlaxoSmithKline plc Quote
Zacks Rank & Stocks to Consider
GSK currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
A couple of better-ranked biotech stocks are Alkermes ALKS and Sesen Bio SESN, both sporting a Zacks Rank #1.
The Zacks Consensus Estimate for Alkermes’ 2022 loss per share has narrowed from 14 cents to 3 cents in the past 60 days. Shares of ALKS have risen 18.1% year to date.
Earnings of Alkermes beat estimates in each of the last four quarters, the average being 350.48%.
The Zacks Consensus Estimate for Sesen Bio’s 2022 loss has narrowed from 33 cents to 32 cents per share in the past 60 days. Shares of SESN have declined 10.6% in the year-to-date period.
Earnings of Sesen Bio beat estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 69.94%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
To read this article on Zacks.com click here.
Zacks Investment Research
