ImmunoGen (IMGN) Files BLA for Lead Drug in Ovarian Cancer
ImmunoGen, Inc.’s IMGN shares increased 11.7% after the company announced that it has filed a biologics license application (“BLA”) with the FDA, seeking approval for its lead candidate, mirvetuximab soravtansine, in platinum-resistant ovarian cancer.
The BLA seeks FDA’s approval under the accelerated pathway for mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor (FR) alpha who have already been treated with one to three prior systemic treatments.
The filing was based on data from the pivotal phase III SORAYA study, which evaluated the candidate as monotherapy in the given indication. We remind investors that the company had announced back in November 2021 that the study met its primary endpoint of objective response rate.
ImmunoGen has requested a priority review of the BLA filing, which, if granted, will complete the review within a period of six months from the BLA’s filing date. Mirvetuximab soravtansine was granted fast track and orphan drug designations by the FDA in ovarian cancer indication.
In the year so far, ImmunoGen’s stock price has declined 34.5% in comparison with the industry’s 12.4% decline.
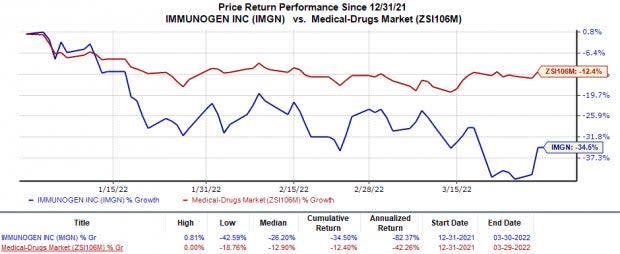
Image Source: Zacks Investment Research
ImmunoGen is currently enrolling patients in the phase III MIRASOL study, which is evaluating mirvetuximab as a monotherapy in patients with platinum-resistant ovarian cancer with FR alpha-high. Data from this study, which is anticipated in third-quarter 2022, will support full approval of mirvetuximab in the given indication.
Currently, IMGN has no marketed drug in its portfolio. Hence, it remains highly dependent on its partners who use the antibody-drug conjugate (ADC) technology for revenues. A potential approval for mirvetuximab soravtansine will reduce the company’s dependence on its partners for revenues.
One such partner is Roche Holding AG RHHBY, whose marketed drug Kadcyla uses the company’s ADC technology. Roche’s Kadcyla is currently approved by the FDA as a prescription drug in early breast cancer and metastatic breast cancer indications. The drug is one of the key drivers of RHHBY’s top line. For full-year 2021, Kadcyla sales were up 16% from 2020.
Last month, ImmunoGen entered into a licensing agreement with Eli Lilly and Company LLY. The agreement will provide Lilly exclusive rights to the company’s technology to develop and commercialize ADCs directed toward targets selected by LLY.
In return, Eli Lilly will pay an upfront payment of $13 million to IMGN for the initial targets. ImmunoGen is also eligible to receive an additional $32.5 million for any additional targets selected by Lilly. ImmunoGen is entitled to receive up to $1.7 billion in potential target program exercise fees and development as well as regulatory and commercial milestone payments.
ImmunoGen, Inc. Price
ImmunoGen, Inc. price | ImmunoGen, Inc. Quote
Zacks Rank & Stock to Consider
ImmunoGen currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector is Collegium Pharmaceutical COLL, which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Collegium Pharmaceutical’s earnings per share estimates for 2022 have increased from $3.79 to $5.76 in the past 60 days. The same for 2023 has increased from $4.79 to $7.96 in the past 60 days. Shares of COLL have risen 8% year to date.
Earnings of Collegium Pharmaceutical beat estimates in three of the last four quarters and missed the mark on one occasion, with the negative surprise being 57.6%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
ImmunoGen, Inc. (IMGN) : Free Stock Analysis Report
Collegium Pharmaceutical, Inc. (COLL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
