Incyte (INCY) Label Expansion for Ruxolitinib Cream Delayed
Incyte Corporation INCY announced that the FDA has extended the review period for the supplemental New Drug Application (sNDA) for ruxolitinib cream.
The sNDA is seeking approval of the cream for the treatment of vitiligo. The regulatory body has extended the target action date by three months to Jul 18, 2022. The sNDA was originally accepted and granted priority review by the FDA in December 2021 and granted a target action date of Apr 18, 2022.
The FDA extended the target action date to review additional data from the ongoing phase III studies submitted by Incyte in response to the FDA’s information request. The FDA has determined the submission of the additional information to constitute a major amendment to the sNDA, resulting in an extension of the target action date.
We remind investors that ruxolitinib cream is a novel formulation of Incyte’s selective JAK1/JAK2 inhibitor ruxolitinib and is marketed under the brand name Opzelura.
The delay in a potential approval is disappointing.
It is the first and only topical JAK inhibitor approved for use in the United States for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis (AD) in non-immunocompromised patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
In October 2021, the Marketing Authorization Application (MAA) for ruxolitinib cream in Europe was validated as a potential treatment for adolescents and adults with non-segmental vitiligo with facial involvement.
We note that a phase III randomized trial is evaluating the efficacy and safety of ruxolitinib cream in children ages two to 12 years with AD is currently ongoing.
A phase III study evaluating ruxolitinib cream in chronic hand eczema is being initiated.
Shares of Incyte have lost 9.7% in the past year compared with the industry’s 40% decline.
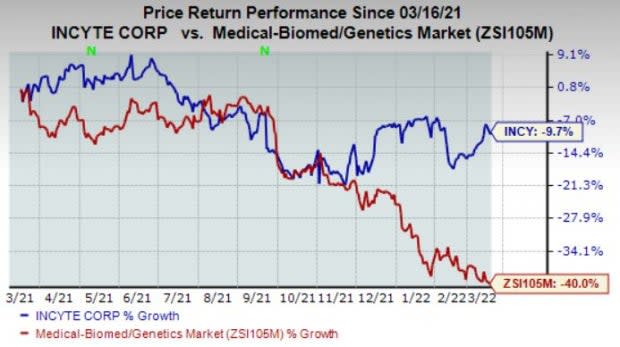
Image Source: Zacks Investment Research
While Jakafi maintains momentum for the company, the recent pipeline setbacks weigh on it. The FDA issued a Complete Response Letter (CRL) for the biologics license application (BLA) for its intravenous PD-1 inhibitor, retifanlimab, for the treatment of adult patients with locally advanced or metastatic squamous cell carcinoma of the anal canal (SCAC) who have progressed on or are intolerant of platinum-based chemotherapy.
Incyte has a collaboration with Novartis NVS for Jakafi. We note that Jakafi is marketed by Incyte in the United States and by Novartis as Jakavi outside the country. However, the agreement with Novartis does not include topical administration.
Incyte currently carries a Zacks Rank #4 (Sell).
A couple of better-ranked stocks in the biotech space are Vertex Pharmaceuticals VRTX and BioDelivery Sciences BDSI, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Vertex Pharmaceuticals’ earnings per share estimates have increased to $14.52 from $13.39 for 2022 over the past 60 days. Shares of Vertex have gained 12% in the past year.
BioDelivery Sciences’ earnings per share estimates for 2022 have increased from 33 cents to 38 cents in the past 30 days. Shares of BDSI have surged 79.7% year to date.
Earnings of BioDelivery Sciences beat estimates in three of the last four quarters, missing the mark once, the average surprise being 33.7%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Incyte Corporation (INCY) : Free Stock Analysis Report
BioDelivery Sciences International, Inc. (BDSI) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
