Incyte's (INCY) BLA for Cancer Drug Accepted for Priority Review
Incyte INCY announced that the FDA has accepted its Biologics License Application (BLA) for pipeline candidate, retifanlimab, an investigational intravenous anti-PD1 antibody.
The company is seeking approval of the candidate as a potential treatment for adult patients with locally advanced or metastatic squamous cell carcinoma of the anal canal (SCAC) who have progressed on or are intolerant to platinum-based chemotherapy.
The agency has granted Priority Review to the application.
We note that the FDA grants Priority Review to medicines that may offer a major advance in the treatment of diseases with unmet needs. This designation shortens the review period by four months as compared to standard review. The target action date for the BLA is Jul 25, 2021.
The BLA submission was based on data from the phase II POD1UM-202 study evaluating retifanlimab in previously treated patients with locally advanced or metastatic SCAC who have progressed on or are intolerant to standard platinum-based chemotherapy.
Results showed an objective response rate (ORR) of 14% for retifanlimab monotherapy as determined by an independent central review (ICR) using RECIST v1.1. Responses were observed regardless of PD-L1 status, presence of liver metastases, age or HIV+ status and were durable (median 9.5 months).
The FDA had earlier granted an Orphan Drug designation to the candidate for the treatment of anal cancer.
Meanwhile, POD1UM-303/InterAACT 2 (NCT04472429), a phase III study of retifanlimab in combination with carboplatin and paclitaxel in patients with inoperable locally recurrent or metastatic SCAC, is now open and enrolling patients.
Incyte entered into an exclusive collaboration and license agreement with MacroGenics, Inc. MGNX for global rights of retifanlimab in 2017.
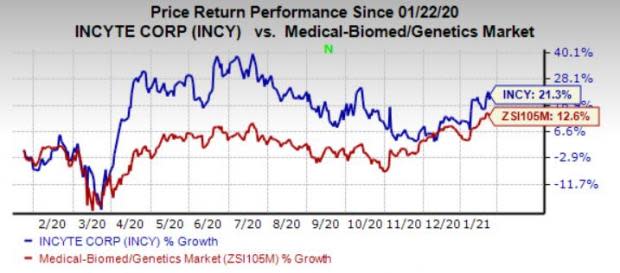
Shares of the company have gained 20.1% in the past year compared with the industry’s 14.9% growth.
Incyte’s lead drug, Jakafi, a first-in-class JAK1/JAK2 inhibitor, is already approved by the FDA for the treatment of polycythemia vera (PV). It is also approved for the treatment of steroid-refractory acute GVHD in adult and pediatric patients 12 years and older.
The drug is marketed by Incyte in the United States and by Novartis NVS, as Jakavi, outside the country.
The company’s efforts to develop its pipeline and diversify the revenue base are encouraging. Moreover, the recent approval of Pemazyre and Monjuvi in combination with MorphoSys’ MOR and Tabrecta (with Novartis) will bring additional sales and diversify the revenue base.
Incyte currently carries a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank stocks here.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2021.
Click here for the 6 trades >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Incyte Corporation (INCY) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
MacroGenics, Inc. (MGNX) : Free Stock Analysis Report
MorphoSys AG Unsponsored ADR (MOR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
