Intellia's (NTLA) NTLA-5001 Gets FDA Orphan Drug Tag for AML
Intellia NTLA announced that the FDA granted Orphan Drug designation (“ODD”) to its first wholly-owned ex-vivo genome editing CRISPR candidate, NTLA-5001, for the treatment of acute myeloid leukemia (“AML”).
The ODD is granted by the FDA to the candidates being developed to treat, diagnose or prevent a rare disease or condition. ODD makes the sponsor eligible to receive seven years of market exclusivity, following the potential approval and tax credit for qualified clinical studies.
Shares of Intellia have lost 44.2% so far this year in comparison with the industry’s 17% decline.
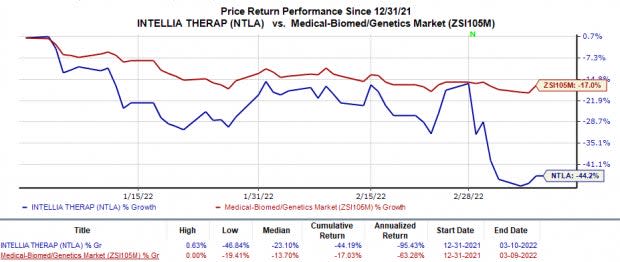
Image Source: Zacks Investment Research
Cancer of the blood and bone marrow, AML is marked by an over-expression of the Wilms’ Tumor (WT1) antigen. NTLA-5001 is a T-cell receptor-based T-cell therapy engineered to target the WT1 antigen to treat all genetic subtypes of AML.
Earlier this month, Intellia announced that it dosed the first patient in a phase I/IIa study, which is evaluating NTLA-5001 in adults with persistent or recurrent AML who previously received first-line therapy.
Apart from NTLA-5001, Intellia is evaluating other investigational CRISPR therapies in its pipeline. The company is developing curative therapeutics using the CRISPR/Cas9 technology. NTLA is evaluating its lead in-vivo genome-editing candidate, NTLA-2001, in a phase-I study for treating transthyretin amyloidosis with polyneuropathy (ATTRv-PN). NTLA-2001 is part of a co-development and co-promotion agreement with Regeneron Pharmaceuticals REGN.
Last month, Intellia and Regeneron announced positive interim data from the above-mentioned phase-I study. The study reported data from four cohorts that evaluated four different doses of NTLA-2001 in ATTRv-PN patients. The patients treated with NTLA-2001 exhibited a dose-dependent reduction in serum TTR levels, achieving mean reductions of 52%, 87%, 86% and 93% at day 28 in the 0.1 mg/kg, 0.3 mg/kg, 0.7 mg/kg, and 1.0 mg/kg dose cohorts, respectively. The company plans to move forward with a fixed dose of 80mg and evaluate the same in a dose-expansion cohort of the phase I study, which is expected to start in first-quarter 2022.
While Intellia is the lead party for NTLA-2001, Regeneron shares 25% of development costs and commercial profits. Intellia and Regeneron are also developing therapies for hemophilia A and hemophilia B.
Intellia also initiated a phase I/II study evaluating NTLA-2002 for treating hereditary angioedema (“HAE”) in December 2021. NTLA-2002 aims to prevent HAE attacks by suppressing the plasma kallikrein activity.
Intellia Therapeutics, Inc. Price
Intellia Therapeutics, Inc. price | Intellia Therapeutics, Inc. Quote
Zacks Rank & Stocks to Consider
Intellia currently carries a Zacks Rank #4 (Sell).
A few better-ranked stocks in the overall healthcare sector are BioDelivery Sciences BDSI and Vertex Pharmaceuticals VRTX, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
BioDelivery Sciences’ earnings per share estimates for 2022 have increased from 33 cents to 35 cents in the past 30 days. Shares of BDSI have surged 80% year to date.
Earnings of BioDelivery Sciences beat estimates in three of the last four quarters and missed the mark once, the average surprise being 33.7%.
Vertex Pharmaceuticals’ earnings per share estimates for 2022 have increased from $14.33 to $14.52 in the past 30 days. Shares of VRTX have risen 8.9% year to date.
Earnings of Vertex Pharmaceuticals beat estimates in each of the last four quarters, the average being 10%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
BioDelivery Sciences International, Inc. (BDSI) : Free Stock Analysis Report
Intellia Therapeutics, Inc. (NTLA) : Free Stock Analysis Report
To read this article on Zacks.com click here.
