J&J (JNJ) COVID-19 Booster Jab Compares Well With Comirnaty
Johnson & Johnson JNJ announced promising preliminary data from an independent study that evaluated the booster dose of its single-shot COVID-19 vaccine, Ad26.COV2.S, administered at six months after a two-dose primary regimen of Pfizer PFE and BioNTech’s BNTX mRNA-based COVID-19 vaccine, Comirnaty (BNT162b2). The study included a subset of participants in a phase II study — COV2008 — that is being sponsored by J&J and is conducted by Beth Israel Deaconess Medical Center.
Data from the study demonstrated that the administration of the booster dose of J&J’s vaccine-elicited neutralizing and binding antibody levels at week four post administration to same after a booster dose of Pfizer and BioNTech’s Comirnaty. Moreover, J&J’s booster dose also resulted in a greater increase in T-cell responses compared to Comirnaty.
Please note that while neutralizing antibodies help in blocking infection, T-cell destroys infected cells.
This preliminary data is also supported by an initial data from another clinical study — UK COV-BOOST — that evaluated J&J’s COVID-19 vaccine’s booster dose in participants who have received immunization with Pfizer and BioNTech’s Comirnaty or AstraZeneca’s AZN COVID-19 vaccine, ChAdOx1 nCov-19, in their primary vaccination regimen. Data from the UK COV-BOOST study also demonstrated an increase in both antibody and T-cell responses following J&J’s booster dose.
We note that AstraZeneca is one of the early players to market a COVID-19 vaccine in the world. However, AZN is yet to receive authorization for its vaccine in the United States. AstraZeneca produced the vaccine at cost amid the pandemic. The vaccine generated $1.05 billion in revenues for AstraZeneca in the first nine months of 2021.
This year so far, J&J’s shares have risen 1.2% compared with an increase of 12.3% for the industry.
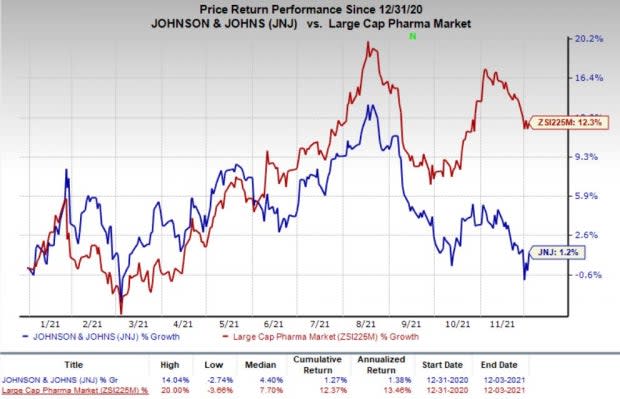
Image Source: Zacks Investment Research
J&J is a late entrant in the COVID-19 vaccine race. The company’s vaccine was first authorized for emergency use in the United States in February 2021. It expects $2.5 billion in revenues from the COVID-19 vaccine in 2021, significantly less than Pfizer’s $36 billion from its COVID-19 vaccine in the same period.
However, J&J’s booster dose was the first to receive emergency use authorization in the United States in October for all adults. Moreover, the recent preliminary data from the phase II study supports the use of J&J’s booster dose in people who have received initial vaccination with Comirnaty for better protection.
We note that the FDA had authorized the use of mix and match or heterologous administration of the booster doses in October that allows the administration of a booster dose of any authorized COVID-19 vaccine, irrespective of the primary vaccination regimen. This authorization of heterologous administration should ease the concerns related to the use of a different booster jab than the initial regimen.
With the majority of the population in the United States having received primary vaccination, the main revenue driver for the COVID-19 vaccine makers will come from the sale of booster doses. Clinical data supporting better protection with J&J’s booster dose may help the company drive the supply of booster doses higher next year. This may result in higher additional revenues for J&J from the COVID-19 vaccine next year.
Johnson & Johnson Price

Johnson & Johnson price | Johnson & Johnson Quote
Zacks Rank
J&J currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
