Juul halts flavored e-cig retail sales, removes social media presence
The nation’s leading e-cigarette manufacturer Juul Labs released plans Tuesday to curtail the underage use of its products after facing mounting pressure from the U.S. Food and Drug Administration.
Meeting the 60-day deadline the FDA imposed upon top e-cig manufacturers to submit plans, Juul announced it would stop selling flavored e-cigarettes to all of the 90,000 retail locations where they are currently sold. The company will continue selling flavored e-cigarettes, which FDA Commissioner Scott Gottlieb has called out for appealing to teenage users, through its website which requires more stringent age verification hurdles.
The company, which has come under fire for appealing to teens through advertisements and other messages on social media, also announced it would be ending its presence on social media by removing its Facebook and Instagram accounts. The company said it would maintain a presence on Twitter for “non-promotional communications” and on YouTube to air its testimonials from former smokers.

“By deterring social media promotion of the Juul system by exiting our accounts, we can better prevent teens and non-smokers from ever becoming interested in the device,” Juul CEO Kevin Burns said in a statement.
Juul’s verified Instagram account had over 77,000 Instagram followers as of Tuesday. The company’s Facebook page had over 10,000 likes from users.
Juul’s move to restrict flavored e-cigarettes at retail locations comes roughly a week after The Washington Post reported the FDA was moving closer to a retail ban on all e-cig flavors except menthol and mint, similar to what traditional cigarette flavors are currently limited to.

The FDA has increasingly been eyeing regulations in the e-cigarette space as teenage use continues to rise. Commissioner Gottlieb often cites a 77% rise in e-cig use among high schoolers as a worrying statistic. Other studies, including one from anti-tobacco group Truth Initiative, found underage teens were more likely to use Juul than 25- to 34-year-olds.
Just a temporary sales halt
Pending the final ruling on what the FDA deems acceptable moving forward, Juul said it could reinstate the sale of its flavors, including fruit, mango, and cucumber, only at retail locations that establish point-of-sale age verification technology. Such a process would require the physical scanning of I.D.s to prove a customer is over the age of 21, regardless of local age requirements that may be less stringent. The company also reiterated its support for raising the tobacco purchasing age to 21 in areas where it is only 18.
The decision will have a major impact on the overall e-cigarette market. Since growing its annual sales rate to $1.5 billion, Juul has come to control roughly 75% of the e-cigarette market, according to Nielsen sales data compiled by Wells Fargo analyst Bonnie Herzog. As a result of its exponential sales growth, Juul also became the quickest company in history to earn a valuation of more than $10 billion – just seven months after its first funding round.
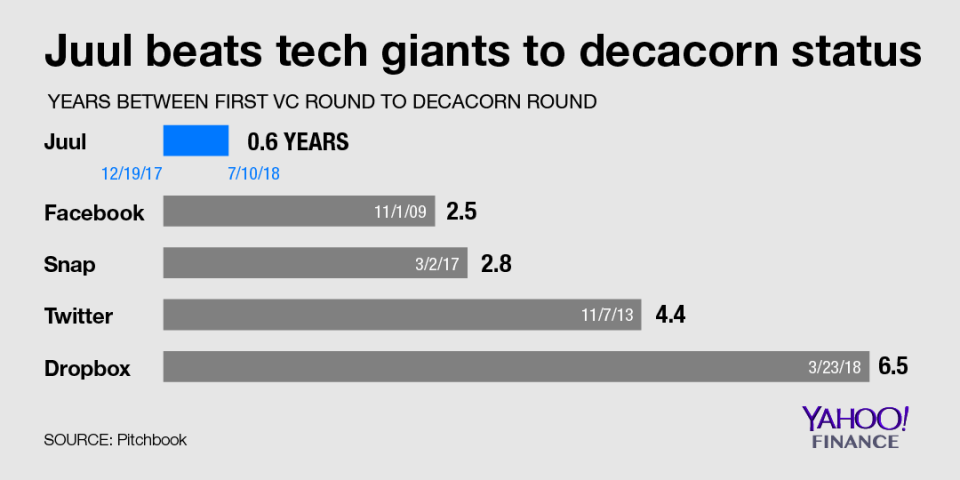
In the statement outlining the company’s plans, Burns reiterated Juul is a smoking cessation product meant to be targeted at adult smokers.
“Our intent was never to have youth use Juul,” he said. “But intent is not enough, the numbers are what matter, and the numbers tell us underage use of e-cigarette products is a problem. We must solve it.”
Commissioner Gottlieb and the FDA have yet to weigh in on whether the latest move will be enough to solve the issue of teenage e-cigarette use he has labeled an “epidemic,” but considering it is in-line with what the agency was reportedly already considering, it’s seemingly a lock-step move in the right direction.
Zack Guzman is a senior writer and on-air reporter covering entrepreneurship, startups, and breaking news at Yahoo Finance. Follow him on Twitter @zGuz.
Read more:
Juul surpasses Facebook as fastest startup to reach decacorn status
How Juul became the FDA’s latest target
Joe Camel illustrator: E-cig maker Juul’s marketing ‘seems more egregious’
