MATEON THERAPEUTICS AND WINDLAS BIOTECH ENTER INTO DEFINITIVE AGREEMENT TO COMMERCIALZE ARTIVEDA™ AGAINST COVID-19
Mateon Therapeutics
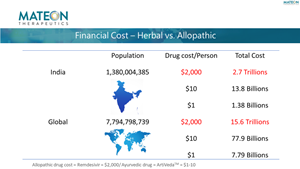
Financial Cost - Herbal vs. Allopathic
AGOURA HILLS, Calif., Nov. 11, 2020 (GLOBE NEWSWIRE) -- Mateon Therapeutics (OTCQB: MATN), a leading developer of TGF-β therapeutics, announced today an agreement with Windlas Biotech Pvt. Ltd. of India (Windlas) to commercialize ARTIVeda™. ARTIVeda™ is Mateon’s lead ethnobiology drug against COVID-19.
With clinical data supporting anti-viral activity in-vitro and in-vivo, and with its high safety index, the commercial path has been accelerated both as an Ayurvedic therapy and Nutraceutical in India that can be prescribed by physicians. We, with and through our commercialization partners Windlas, are in active discussion with large distributors, marketers, and manufacturers to establish a consortium for distribution of this drug product in an equitable manner across all territories.
Early data from ARTI-19 suggests efficacy trend and safety, which is further supported by an independent recently completed study published in Int J Antimicrob Agents. 2020 Nov 2. In this study, time to undetectable SARS-CoV-2 RNA in the treatment group was significantly less than the control group (Treatment: 10.6±1.1 days), Control: 19.3±2.1 days) and Length of hospital stay for Treatment group was 13.3±4.8 days, and Control was 21.3±9.1 days.
Windlas is a leading CDMO since last 20 years. It promotes more than 120 chronic and acute care branded products (allopathic, nutraceutical and Ayush formulations) through its “affordable generics platform” spanning over 950 wholesalers across India. Windlas branded medicines and wellness products are sold in several markets across the globe like Sri Lanka, Vietnam, Thailand, Myanmar etc.
In order to maximize the accessibility and the reach of “ARTIVeda™”, Mateon and Windlas intend to launch it through its distribution network as well as through strong co-marketing partners who are existing clients of Windlas.
“We are very excited by the early data of our Phase IV Clinical trial in Indian COVID-19 patients, and confirmatory data of other global researchers. Given the well-established safety profile of this product, with hundreds of years of usage against viral fever and associated symptoms, ARTIVeda™ has the potential for use even beyond the pandemic as a safe and effective anti-viral therapy,” said Hitesh Windlass, Managing Director of Windlas. “Windlas has the capability to manufacture and supply several hundred million doses of ARTIVeda™ a month to address the dire patient needs.”
Saran Saund, Chief Business Officer and GM of AI division of Mateon, commented, “Signing the definitive agreement with Windlas is a significant milestone for the companies to come together to address this epidemic in India and rest of world. We look forward to this partnership having an impact that can make slow down or even stopping this pandemic. Windlas, with its world-class manufacturing and commercialization operations and Mateon’s innovative science team create a formidable partnership”.
“We are very excited to have signed this milestone agreement and look forward to a long and successful partnership with Windlas. We believe that ARTIVeda™ can be a potent solution to the pandemic and the potential revenues from the sale of ARTIVeda™ could be significant,” said Amit Shah, CFO for Mateon. “We anticipate launching ARTIVeda™, initially in India through our commercialization partner Windlas, and then globally, reaching out to as many people as possible in need of a drug for COVID-19, which is close to becoming the most widespread virus in the history of mankind.”
About Windlas Biotech Pvt. Ltd, India
Windlas Biotech Private Limited is a 20-year-old company with a strong track record of research, development, manufacturing and distribution of pharmaceutical products in India, USA and several other emerging markets. It has four large scale manufacturing facilities employing more than 1500 employees and is the 5th largest Contract Development and Manufacturing Organization (CDMO) serving top innovator as well as generic pharma companies across the world. It has developed more than 500 different formulations (> 2Billion doses annually) of anti-viral, cardiovascular, anti-diabetic, anti-infective, CNS and dermatology products.
About ARTIVeda™ and ArtiShield™
ARTIVeda is Ayurveda - Dvipaantara Damanaka- and is labeled as capsule containing Artemisia Powder 500mg. Ayurveda is a natural system of medicine, originated in India more than 3,000 years ago. Its use in this trial to treat COVID-19 is per Ayurvedic text: fever and inflammation. Artemisinin is an active component of ARTIVeda™. Artemisinin is able to inhibit TGF-β activity and is able to neutralize SARS-CoV-2 (COVID-19) in vitro at an EC50 of 0.45 ug/ml (based on Mateon’s test result at Utah State University), and a Safety Index of 140, which is superior to remdesivir and chloroquine. The unpurified herb extract has no anti-viral activity. ARTIVeda™ is designed to target multiple viral threats including COVID-19 by suppressing both viral replication and clinical symptoms that arise from viral infection. A phase IV trial looking at ARTIVeda™ in COVID-19 is ongoing in India and globally. The US name for this drug product is ArtiShield™. We are looking to leverage ex-US data for the commercialization of ArtiShield™ in the US. We are expecting ArtiShield™ to be cost effective prophylactic suitable for global deployment.
About Mateon Therapeutics
Mateon was created by the recent reverse merger with Oncotelic, which became a wholly owned subsidiary of Mateon, thereby creating an immuno-oncology company dedicated to the development of first in class RNA therapeutics as well as small molecule drugs against cancer and infectious diseases. OT-101, the lead immuno-oncology drug candidate of Mateon/Oncotelic, is a first-in-class anti-TGF-βRNA therapeutic that exhibited single agent activity in some relapsed/refractory cancer patients in clinical trial settings. OT-101 also has activity against SARS-CoV-2. Mateon/Oncotelic is seeking to leverage its deep expertise in oncology drug development to improve treatment outcomes and survival of cancer patients with a special emphasis on rare pediatric cancers. Mateon has rare pediatric designation for DIPG (OT-101), melanoma (CA4P), and AML (OXi4503). For more information, please visit www.oncotelic.com and www.mateon.com.
Mateon's Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this communication regarding strategy, future operations, future financial position, prospects, plans and objectives of management are forward-looking statements. Words such as “may”, “expect”, “anticipate” “hope”, “vision”, “optimism”, “design”, “exciting”, “promising”, “will”, “conviction”, "estimate," "intend," "believe", “quest for a cure of cancer”, “innovation-driven”, “paradigm-shift”, “high scientific merit”, “impact potential” and similar expressions are intended to identify forward-looking statements. Forward-looking statements contained in this press release include, but are not limited to, statements about future plans, the progress, timing, clinical development, scope and success of future clinical trials, the reporting of clinical data for the company’s product candidates and the potential use of the company’s product candidates to treat various cancer indications. Each of these forward-looking statements involves risks and uncertainties and actual results may differ materially from these forward-looking statements. Many factors may cause differences between current expectations and actual results, including unexpected safety or efficacy data observed during preclinical or clinical studies, clinical trial site activation or enrollment rates that are lower than expected, changes in expected or existing competition, changes in the regulatory environment, failure of collaborators to support or advance collaborations or product candidates and unexpected litigation or other disputes. These risks are not exhaustive, the company faces known and unknown risks, including the risk factors described in the company’s annual report on Form 10-K filed with the SEC on April 10, 2019 and in the company’s other periodic filings. Forward-looking statements are based on expectations and assumptions as of the date of this press release. Except as required by law, the company does not assume any obligation to update forward-looking statements contained herein to reflect any change in expectations, whether as a result of new information future events, or otherwise.
Contact Information:
For Mateon Therapeutics, Inc.:
Amit Shah
ashah@oncotelic.com
Attachment
