Medtronic (MDT) Gets FDA's 510(k) Nod for Pediatric ICM
Medtronic MDT recently received FDA’s 510(k) clearance for its new-generation LINQ II Insertable Cardiac Monitor (ICM) system for pediatric patients. This latest development is a landmark for Medtronic considering the fact that, it is the first-and-only ICM for use in pediatric patients (over the age of 2) with heart rhythm abnormalities to receive 510(k) clearance.
Demand for devices like ICM is gaining day by day with growing prevalence of cardiac rhythm abnormalities among pediatric patients in recent times. This latest approval broadens Medtronic’s scope within its Cardiovascular Diagnostics and Services wing within broader Cardiovascular business.
More About the Approval
LINQ II ICM is a small (one-third the size of a AAA battery), wireless ICM for patients with abnormal heart rhythms. This is effective on patients who experience infrequent symptoms including dizziness, palpitations, syncope and chest pain, and require long-term monitoring or ongoing management.
LINQ II ICM has a battery life of up to 4.5 years It allows patients to undergo magnetic resonance imaging (MRI) whenever required. This implantable device, can be well carried during daily activities like showering, bathing, or swimming.
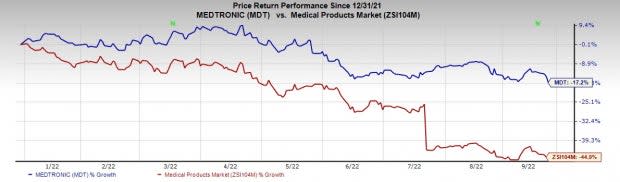
Image Source: Zacks Investment Research
The device was first commercialized in 2020 and has been implanted in thousands of patients globally since then.
The LINQ II ICM system operates together with MDT’s recently launched AccuRhythm AI algorithms. During the process, AccuRhythm applies artificial intelligence (AI) to heart rhythm event data collected by the LINQ II ICM thereby improving the accuracy of patient information to better diagnose and treat abnormal heart rhythms.
Market Prospects
Going by a Facts & Factors report published Globenewswire, global Cardiac Rhythm Management Devices Market size & share in terms of revenue was valued at $17,274.6 million in 2021 and is expected to surpass around $24,071.19 million by 2028, growing at a CAGR of approximately 6.3%.
The latest development accordingly, seems well-timed and strategic for Medtronic.
Medtronic Gains Market Share in Cardiovascular
Medtronic is strongly expanding its global foothold within the company’s Cardiovascular business (consisting of more than 36% of the company’s total revenues in 2022). Within Cardiovascular, cardiac rhythm management, one of Medtronic’s largest businesses, continued to build on the company’s category leadership. Within cardiac rhythm management, Medtronic’s pacing business continued to outperform the market banking on strong global growth of its Micra leadless pacemaker family as it enters new geographies and expands penetration in existing markets. Micra grew 15% in fiscal Q1, including high 70s growth in Japan, mid-teens growth in Western Europe, and high 30s growth in emerging markets.
Further, ICDs (Implantable cardioverter-defibrillator) within cardiac rhythm management are expected to gain significant market share with the upcoming launch of Aurora extravascular ICD. The company currently waiting for the CE Mark approval for AURORA, and expects the U.S. approval in the next calendar year.
Within Structural Heart, the company looks forward to market share gain on strong Transcatheter aortic valve replacement (TAVR) prospects. According to Medtronic, TAVR is one of the largest growth drivers for the company and it expects the market, which is roughly $5.5 billion today, to exceed $7 billion within the next three years and reach $10 billion in the next five years. In the United States, Medtronic is currently planning for the full market release of its next-generation TAVR valve, Evolut FX in the second half of fiscal 2023.
Price Performance
Shares of the company have lost 17.2% in a year compared with the industry's fall of 44.9%.
Zacks Rank and Key Picks
Currently, Medtronic carries a Zacks Rank #4 (Sell).
A few better-ranked stocks in the broader medical space that investors can consider are AMN Healthcare Services, Inc. AMN, ShockWave Medical, Inc. SWAV and McKesson Corporation MCK.
AMN Healthcare has a long-term earnings growth rate of 3.2%. The company surpassed earnings estimates in the trailing four quarters, delivering a surprise of 15.7%, on average. It currently flaunts a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
AMN Healthcare has outperformed its industry in the past year. AMN has lost 6.1% against the industry’s 37% fall.
ShockWave Medical, sporting a Zacks Rank #1 at present, has an estimated growth rate of 33.1% for 2023. The company’s earnings surpassed estimates in all the trailing four quarters, the average beat being 180.1%.
ShockWave Medical has outperformed its industry in the past year. SWAV has gained 35% against the industry’s 32.6% fall in the past year.
McKesson has an estimated long-term growth rate of 9.9%. The company surpassed earnings estimates in the trailing three quarters and missed in one, delivering a surprise of 13%, on average. It currently carries a Zacks Rank #2 (Buy).
McKesson has outperformed its industry in the past year. MCK has gained 71.1% against the industry’s 15.1% fall.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Medtronic PLC (MDT) : Free Stock Analysis Report
McKesson Corporation (MCK) : Free Stock Analysis Report
AMN Healthcare Services Inc (AMN) : Free Stock Analysis Report
ShockWave Medical, Inc. (SWAV) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
