Merck's (MRK) COVID Pill Gets FDA Nod for Emergency Use
Merck MRK and partner Ridgeback Biotherapeutics announced that the FDA granted an Emergency Use Authorization (EUA) to molnupiravir, its oral antiviral candidate for treating high-risk adults with mild-to-moderate COVID-19. However, the drug can be prescribed to only those patients for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate.
Molnupiravir can also be not prescribed for use for more than five consecutive days. Moreover, the drug cannot be prescribed to pregnant women or patients below the age of 18.
The drug is not granted an EUA to prevent COVID-19 or initiate treatment in patients hospitalized due to COVID.
The FDA authorization was expected as the FDA’s Antimicrobial Drugs Advisory Committee had already voted in favor of authorizing molnupiravir for the given indication in November 2021.
The authorization of molnupiravir was based on data from the phase III MOVe-OUT study. Data from the final analysis of the MOVe-OUT study, announced in November, showed that the medicine reduced the risk of hospitalization or death by approximately 30% in non-hospitalized at-risk adult patients with mild or moderate COVID-19, which was less than 50% as previously reported, as part of the interim data announced in October.
Final data from all enrolled participants from the study showed that 6.8% of patients treated with molnupiravir was hospitalized or died versus the 9.7% of placebo-treated patients. There was one death reported in patients treated with molnupiravir versus nine deaths among the placebo-treated patients.
The pill demonstrated antiviral activity against the Omicron variant in preclinical studies.
Merck expects to begin shipping molnupiravir in the coming days. MRK already has a supply agreement for 3.1 million courses of the drug with the United States government. MRK expects to manufacture 10 million courses of molnupiravir by this year-end and at least 20 million courses by 2022 end.
Shares of Merck have declined 7.4% this year so far against an increase of 20% for the industry.
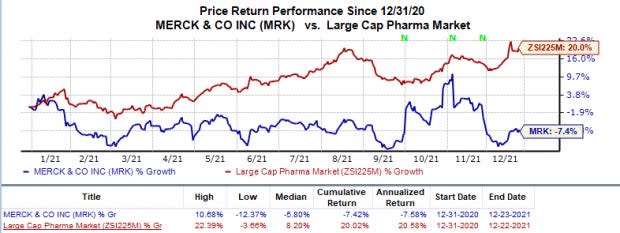
Image Source: Zacks Investment Research
With regard to other countries, molnupiravir received its first authorization for use in the United Kingdom last month. In Europe, the European Medicines Agency began a rolling review of Merck/ Ridgeback Biotherapeutics’ regulatory application for molnupiravir. Regulatory applications seeking authorization for molnupiravir in multiple nations are currently underway.
Merck is also evaluating the drug in the phase III MOVe-AHEAD study for the prevention of COVID-19.
Presently, mild-to-moderate COVID-19 patients are being treated with antibody cocktails developed by companies like Regeneron REGN and Eli Lilly LLY. However, the same needs to be administered in a hospital.
Regeneron’s antibody cocktail REGEN-COV comprises two monoclonal antibodies, namely casirivimab and imdevimab and is approved for the treatment of and post-exposure prophylaxis in certain high-risk individuals. It became a significant contributor to its top line in recent quarters.
Regeneron is in discussion with the FDA to expand the current EUA to other populations, including pre-exposure prevention and hospitalized patient settings. REGN’s antibody cocktail was approved in Europe (known as Ronapreve in the European Union) last month for the treatment as well as prevention of COVID-19.
Lilly’s COVID-19 antibody cocktail bamlanivimab plus etesevimab was granted an emergency approval by the FDA in February 2021 to treat mild-to-moderate COVID-19 in high-risk patients.
In September, the FDA expanded the EUA for the cocktail antibody medicine to include the post-exposure prevention (prophylaxis) for COVID-19 indication. Lilly’s cocktail medicine generated revenues of $217.1 million in the third quarter of 2021.
On Wednesday, the FDA granted an EUA to Pfizer’s PFE Paxlovid, an oral antiviral for COVID-19.Paxlovid is authorized for use in combination with low-dose ritonavir to treat mild-to-moderate COVID-19 in adult and pediatric patients (12 years and older and weighing at least 40 kg) at an increased risk of hospitalizations or death.
The EUA granted to Pfizer’s Paxlovid was based on clinical data from an interim analysis of a phase II/III study named EPIC-HR. Data from the study showed that Paxlovid (administered) reduced the risk of hospitalization or death by 89% in non-hospitalized high-risk adult patients with COVID-19 compared to placebo.
Paxlovid and molnupiravir are antiviral medicines, which can be prescribed as at-home treatments to help fight the mild-to-moderate COVID-19 infection. It can help prevent hospitalization in patients with a mild-to-moderate form of the disease but at high risk of severe COVID-19 and thus lower some of the pressure facing the healthcare and hospital systems.
Amid the rising infection rates and the rapid spread of the Omicron variant across the United States, most monoclonal antibody drugs appear largely ineffective against the pandemic. Both molnupiravir and Pfizer’s Paxlovid are thus considered crucial to fight the crisis and reduce pressure on the hospitals.
Merck & Co., Inc. Price

Merck & Co., Inc. price | Merck & Co., Inc. Quote
Zacks Rank
Merck currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
