Moderna Rallies on Encouraging Coronavirus Vaccine Study Data
Moderna, Inc. MRNA announced positive interim data from a phase I study evaluating its mRNA-based coronavirus vaccine candidate, mRNA-1273. Data demonstrated that patients vaccinated with mRNA-1273 achieved levels of antibodies similar or higher than those typically found in a patient who recovered from COVID-19 naturally. The study is being conducted by the National Institute of Allergy and Infectious Diseases.
Moreover, updated pre-clinical data has showed that mRNA-1273 prevented viral replication in the lungs in a mouse challenge model. The candidate was also granted Fast Track designation earlier this month. This will likely expedite the review of a potential regulatory application seeking approval for mRNA-1273.
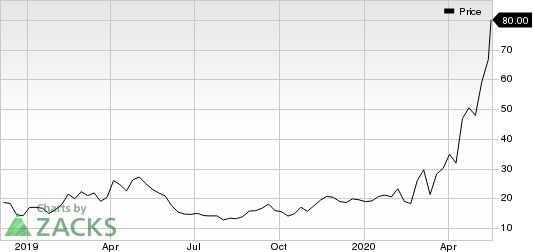
Shares of Moderna rose 20% on Monday, following the encouraging study data on the coronavirus vaccine candidate. In fact, the company’s shares have skyrocketed 309% so far this year compared with the industry’s increase of 8.7%, primarily due to the company’s progress with its coronavirus vaccine candidate.

The ongoing phase I study on mRNA-1273 is evaluating three doses of the candidate — 25 µg, 100 µg and 250 µg — in three patient groups — adults aged 18 to 55 years, older adults aged 56 to70 years and elderly people aged 71 years and above — in nine separate cohorts.
Across all doses, all patients were seroconverted (developed specific antibodies against COVID-19 and the antibodies became detectable in the blood) by day 15 after a single dose. Meanwhile, patients after receiving two 25 µg doses of mRNA-1273 achieved levels of binding antibodies seen in convalescent sera (level of antibodies seen in blood sample of patients naturally recovered from COVID-19) at day 43. The patients who received two 100 µg doses achieved levels of binding antibodies significantly exceeding the levels seen in convalescent sera. Moreover, both 25 µg and 100 µg doses achieved levels of neutralizing antibodies at or above levels generally seen in convalescent sera.
On its first-quarter earnings call, Moderna stated that the FDA has reviewed its investigational new drug application for initiating a phase II study on mRNA-1273 and allowed the same. The company plans to start the study soon. The study will evaluate 50 µg and 100 µg doses of mRNA-1273 with a dose to be selected for pivotal studies.
Moreover, the company is finalizing the design for a phase III study on mRNA-1273 and anticipates to initiate the study in July.
The company signed an agreement last month with the Biomedical Advanced Research and Development Authority ("BARDA") under which the agency committed to pay up to $483 million to accelerate the development of its mRNA-based coronavirus vaccine candidate, mRNA-1273. The award will fund the development of the candidate through to FDA licensure.The company signed a strategic collaboration agreement with Lonza for manufacturing of mRNA-1273 earlier this month.
With coronavirus infecting more than two million people worldwide and killing more than145,000, pharmaceutical and biotech companies are racing against time to successfully develop a treatment or vaccine to combat the disease.
Sanofi SNY and Translate Bio TBIO have also collaborated to develop a mRNA vaccine for COVID-19. Sanofi and Glaxo GSK have agreed to co-develop a novel adjuvant coronavirus vaccine using their proven technologies. Other major pharma companies developing vaccine for COVID-19 include Pfizer and J&J.
Zacks Rank
Moderna currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Stocks Set to Double
Each was hand-picked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2020. Each comes from a different sector and has unique qualities and catalysts that could fuel exceptional growth.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Click to get this free report Sanofi (SNY) : Free Stock Analysis Report Moderna, Inc. (MRNA) : Free Stock Analysis Report GlaxoSmithKline plc (GSK) : Free Stock Analysis Report Translate Bio, Inc. (TBIO) : Free Stock Analysis Report To read this article on Zacks.com click here.
