Novavax (NVAX) COVID Jab Gets Expanded Label in WHO's EUL
Novavax, Inc. NVAX announced that the World Health Organization (“WHO”) updated the emergency use listing (“EUL”) of its COVID-19 vaccine, NVX-CoV2373.
Following this update, NVX-CoV2373 is now included in WHO’s EUL for use as a primary series in adults and adolescents aged 12 years and older. The vaccine is now also approved by WHO in adults aged 18 years and older as a booster dose.
Novavax’s COVID vaccine was granted EUL by WHO last year in December for use as a primary series in adults.
The EUL by WHO assesses and lists unlicensed vaccines, therapeutics and in vitro diagnostics to expedite their availability to people affected by a public health emergency. The medications and treatments that are granted EUL meet WHO’s standards for quality, safety and efficacy. Following the grant of these EULs, Novavax’s COVID vaccine is now pre-qualified for supply to numerous WHO member nations.
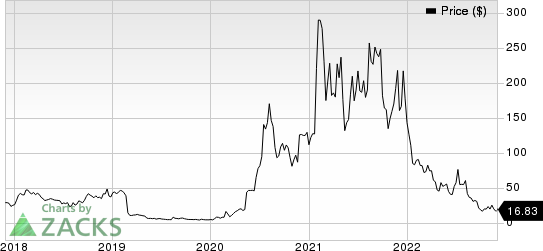
Shares of Novavax have slumped 88.2% this year compared with the industry’s 19.9% fall.

Image Source: Zacks Investment Research
The EUL expansion to Novavax’s COVID vaccine in the adolescent population is based on data from the ongoing pediatric expansion of the phase III PREVENT-19 study, which evaluated NVX-CoV2373 in adolescents. Data from the study demonstrated 80% overall clinical efficacy in adults at the time when the Delta variant was dominating in the United States.
The EUL for the booster dose is based on data from multiple phase II studies conducted in Australia, South Africa and the U.K., which evaluated a booster dose of NVX-CoV2373 in adult participants. Data from these studies showed that a third dose of the vaccine generated increased immune responses compared with the data observed in late-stage studies evaluating Nuvaxovid. The vaccine also induced a robust antibody response when used as a heterologous booster dose.
Some other COVID vaccines which have been granted EUL by WHO include Moderna MRNA and Pfizer PFE/BioNTech BNTX. from their COVID vaccine product sales.
The Pfizer/BioNTech and Moderna vaccines have received full approval for adults in the United States. While Moderna’s vaccine is approved for adults aged 18 years and older, the Pfizer/BioNTech vaccine is approved for individuals aged 12 years and older. These vaccines are also authorized for individuals aged six months and above in the United States.
With regard to booster doses, Moderna and Pfizer/BioNTech have also secured authorization from the FDA for using their respective Omicron BA.4/BA.5-adapted bivalent vaccines in individuals as young as five years of age and older. Novavax is yet to make a regulatory filing with the FDA for its own Omicron-based COVID vaccine.
Novavax, Inc. Price
Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
