Novavax (NVAX) Further Delays FDA Filing for COVID-19 Vaccine
Novavax NVAX announced that it has completed the submission of the final data package — including the complete chemistry, manufacturing and controls (CMC) data module — with the FDA for its protein-based COVID-19 vaccine, NVX-CoV2373.
The CMC submission is a prerequisite for the filing of emergency use authorization (EUA) with the FDA. Novavax expects to file EUA for NVX-CoV2373 with the FDA by this month. Per FDA guidance, the EUA application is required to be submitted at least one month after the filing of the final data package to the FDA.
Once again, Novavax has delayed the filing of its EUA for its COVID vaccine with the FDA. Earlier in May 2021, Novavax announced that its plans to file for the authorization for Novavax in the United States in second-quarter 2021 were delayed to the third quarter. The plan was further delayed in August 2021, when Novavax announced plans to delay this filing to fourth-quarter 2021.
Such delays have been detrimental to Novavax’s stock. In fact, the company’s shares declined 7.6% in the trading session on Friday, as the company extended the filing by one more month to January 2022.
The stock decline could also be attributed to an article from Politico, which stated that the Biden administration officials are concerned about Novavax’s ability to produce enough doses of COVID vaccine in 2022. Last October, Politico issued an article that claimed that Novavax was facing core manufacturing problems related to its COVID-19 vaccine.
Shares of Novavax have rallied 26.6% in the trailing 12 months against the industry’s 21.9% decline.
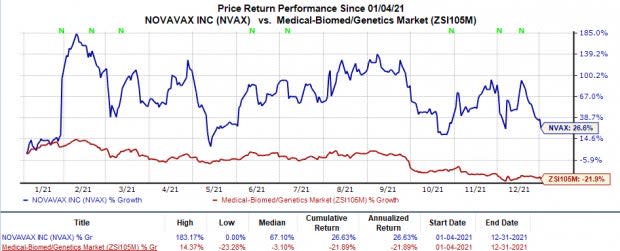
Image Source: Zacks Investment Research
We note that NVX-CoV2373 has already received approval for emergency use in multiple countries outside the United States. Novavax is currently marketing two versions of NVX-CoV2373 — one marketed in partnership with the Serum Institute of India (SII) under the trade name Covovax and another version produced by NVAX that is marketed under the trade name Nuvaxovid.
While Covovax is already authorized for emergency use in India, Indonesia and the Philippines, Nuvaxovid was granted conditional marketing authorization in the European Union (EU) last month.
Please note that Novavax already has an advanced purchase agreement with the European Commission to supply up to 200 million doses of Nuvaxovid. In fact, the member states have already ordered around 27 million doses of the vaccine for first-quarter 2022. The initial doses are expected to arrive in the EU in January.
Last month, the World Health Organization granted emergency use listing (EUL) to both Covovax and Nuvaxovid. Following the grant of EUL, the vaccine is now pre-qualified for supply to numerous countries participating through the COVAX facility. Novavax and SII already have an agreement to jointly supply 1.1 billion doses of NVX-CoV2373 to Gavi through the COVAX facility.
Meanwhile, Novavax has submitted regulatory filings seeking approval for the NVX-CoV2373 in multiple markets like Australia, Canada, Japan, New Zealand, South Korea, UAE and the United Kingdom. Novavax has already entered into advance purchase agreements with many of these nations for supplying doses of its COVID vaccine. A potential approval in any of these markets will give an impetus to the top line.
Although Novavax’s COVID vaccine shows promise and is currently garnering approvals across multiple nations, the company is lagging behind due to stiff competition posed by pharma giants J&J JNJ, Moderna MRNA and Pfizer PFE/BioNTech, which have developed their own COVID-19 vaccines.
We note that while the vaccines developed by Moderna and Pfizer/BioNTech are based on the mRNA technology and require a two-dose primary regimen, the vaccine developed by J&J is an anadenovirus-based vaccine only requiring a single-shot as primary regimen. The vaccines developed by these companies are not only approved for emergency use in the United States but also authorized for use in many countries.
In fact, Pfizer, J&J and Moderna have also been granted EUA for the booster or third dose of their COVID vaccines by the FDA in all adults. Last month, the U.S. Centers for Disease Control and Prevention (“CDC”) recommended the use of mRNA-based COVID-19 vaccines — developed by Moderna and Pfizer — or their booster dose over J&J’s COVID-19 vaccine.
The CDC stated that J&J’s vaccine or its booster dose should be used only when mRNA-based vaccines are contraindicated for a person or are inaccessible.
Novavax, Inc. Price
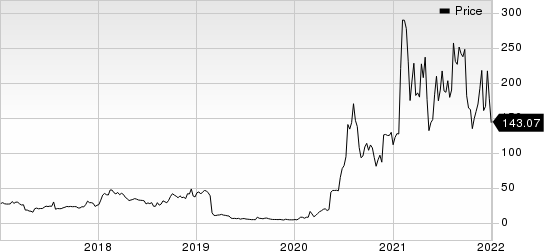
Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
