Novavax (NVAX) Rises Despite U.S. Coronavirus Vaccine Study Delay
Novavax, Inc. NVAX announced that the pivotal phase III study evaluating its COVID-19 vaccine candidate, NVX-CoV2373, in the United States and Mexico, is expected to begin in the coming weeks. The study was earlier expected to begin by the end of November.
The company also announced that the phase III study on NVX-CoV2373 in the United Kingdom and the phase IIb efficacy study in South Africa have completed full enrollment.
Following the delay in the timeline of the U.S./Mexico study on the COVID-19 vaccine candidate, shares of Novavax fell in early trading on Monday. However, the stock rebounded and was up 11% on Monday.
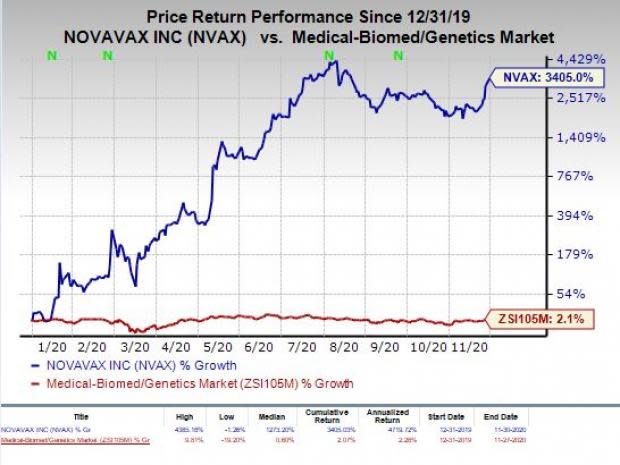
Shares of Novavax have skyrocketed 3405% so far this year compared with the industry’s increase of 2.1%.
We remind investors that in September 2020, Novavax initiated the first phase III study, evaluating the safety, efficacy and immunogenicity of NVX-CoV2373 in the United Kingdom.
In October 2020, the company expanded the study to 15,000 volunteers. The company has now achieved the full-enrollment target in the study and interim data from the same is expected in the first quarter of 2021.
We note that data from the study will support the licensure application for NVX-CoV2373 in the United Kingdom, European Union as well as other countries.
Additionally, the phase IIb study evaluating the efficacy of NVX-CoV2373 in South Africa is also now fully enrolled with 4,422 volunteers. Notably, funding for the manufacturing of doses of NVX-CoV2373 was provided by the Coalition for Epidemic Preparedness Innovations for this study.
Meanwhile, the phase III study in the United States and Mexico is being conducted under the U.S. government’s Operation Warp Speed which has already provided $1.6 billion in funding for delivery of millions of doses of COVID-19 vaccine, if approved. NVX-CoV2373 has been granted a Fast Track designation by the FDA.
Per the press release, Novavax is looking to use vaccine material manufactured at commercial scale for the above-mentioned study.
Notably, the race to develop a safe and effective COVID-19 vaccine has really intensified over the last couple of weeks.
Pfizer PFE and its Germany-based partner BioNTech BNTX have submitted their request for an Emergency Use Authorization (“EUA”) for their mRNA-based coronavirus vaccine candidate, BNT162b2, to the FDA in November.
On Monday, rival vaccine maker Moderna MRNA filed to the FDA, requesting an EUA for its novel mRNA-based coronavirus vaccine candidate, mRNA-1273. Though several other companies are also developing coronavirus vaccine candidates, as of now it seems BNT162b2 and mRNA-1273 are leading the race to get an EUA for their respective vaccine candidates.
Zacks Rank
Novavax currently has a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Legal Marijuana: An Investor’s Dream
Imagine getting in early on a young industry primed to skyrocket from $17.7 billion in 2019 to an expected $73.6 billion by 2027.
Although marijuana stocks did better as the pandemic took hold than the market as a whole, they’ve been pushed down. This is exactly the right time to get in on selected strong companies at a fraction of their value before COVID struck. Zacks’ Special Report, Marijuana Moneymakers, reveals 10 exciting tickers for urgent consideration.
Download Marijuana Moneymakers FREE >>
Click to get this free report Pfizer Inc. (PFE) : Free Stock Analysis Report Moderna, Inc. (MRNA) : Free Stock Analysis Report Novavax, Inc. (NVAX) : Free Stock Analysis Report BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report To read this article on Zacks.com click here. Zacks Investment Research
