Novavax (NVAX) Starts COVID-19 Booster Study in Adolescents
Novavax NVAX announced that it has started evaluating the safety and immunogenicity of a booster/third dose of NVX-CoV2373, its protein-based COVID-19 vaccine, for the pediatric expansion of the phase III PREVENT-19 study in adolescents aged between 12 years and 17 years.
Initial data from this pediatric expansion study is anticipated during second-half 2022. The objectives of the study will include an assessment of the humoral immune response in participants after 28 days of receiving the third dose as well as describing the COVID-19 disease.
The pediatric expansion study had previously evaluated a primary two-dose regimen of NVX-CoV2373 in adolescents. In February, management announced that the study had achieved its primary effectiveness endpoint of NVX-CoV2373, generating neutralizing antibodies in adolescents, similar to the antibody responses noticed in young adult patients (aged between 18 years and 26 years) who were administered the vaccine in the late-stage PREVENT-19 study.
All the earlier adolescent participants who had received a two-dose regimen of the Novavax vaccine are eligible to receive the booster dose of NVX-CoV2373 in this pediatric expansion study. The booster dose is expected to be administered after five months of receiving the second dose of the vaccine.
Shares of Novavax have plunged 67.4% so far this year compared with the industry’s 18.4% decline.
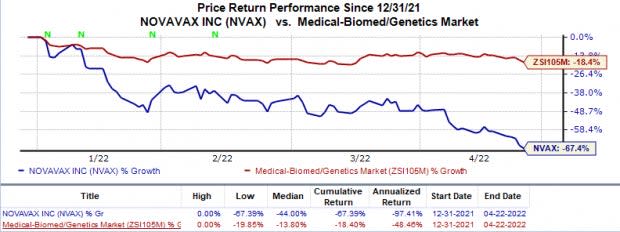
Image Source: Zacks Investment Research
A primary two-dose regimen of NVX-CoV2373 is currently authorized for use in adults in multiple markets, including Australia, Canada, Europe and India.
Novavax started filing regulatory applications seeking authorization for the use of the primary regimen of its COVID-19 vaccine in adolescents. The vaccine received its very first authorization for adolescents in India last month.
However, NVX-CoV2373 is not yet authorized for use in the United States. Though NVAX submitted a regulatory application in January this year to the FDA, seeking an emergency use of NVX-CoV2373 in adults, a final decision by the regulatory agency is yet to be taken.
Currently, the COVID-19 vaccine market in the United States is being dominated by pharma giants like Moderna MRNA and Pfizer PFE/BioNTech BNTX with NVAX trailing way behind.
The vaccines developed by Moderna and Pfizer/BioNTech are based on the mRNA technology. In fact, the two vaccines developed by both companies are currently the only ones that received a full approval for use in adults in the United States. The booster doses of these vaccines are also authorized for use in adults.
The primary regimen of Pfizer/BioNTech’s COVID vaccine is currently the only one authorized for use in individuals aged five years and above in the United States. The third dose of the Pfizer/BioNTech vaccine is also the only one allowed for use in individuals aged 12 years and above.
Although Moderna’s vaccine is yet to be authorized for use in individuals under 18 years of age (either as primary regimen or a booster dose) in the United States, its management already submitted regulatory applications in this regard, which are currently under the FDA review.
Novavax, Inc. Price

Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
