Orchid Ventures Subsidiary PurTec Announces the Launch of PurCore R1 Mesh Coil Cartridge Technology
VANCOUVER, WA / ACCESSWIRE / December 17, 2020 / ORCHID VENTURES, INC. (CSE:ORCD)(OTC PINK:ORVRF) (the "Company" or "Orchid"), a multi-state cannabis innovation company, announces that its wholly-owned subsidiary, PurTec Delivery Systems has launched a revolutionary technological advancement in 510-thread vape cartridges, the most popular form-factor in the vape market. PurCore R1 has been another joint development project with their strategic partner, JWEI Group Advanced Technology Research Institute, built on the JWEI µKera NC technology platform. The Company believes this new technology will set new product safety standards throughout the cannabis vaporizer industry.
PurCore R1 has undergone a ceramic particle safety study where JWEI Group Advanced Technology Research Institute measured ceramic particles in cartridges from the company's leading competitors and compared it against the new PurCore R1 heating element. Ceramic particles are a rising issue where lower quality ceramic materials are being used, and when under pressure in manufacturing and assembly, the ceramic starts to break down. This also occurs during normal expansion and contraction when heating and cooling the ceramic surfaces. The PurTec Innovation Team in development with the JWEI group, has created a more porous and stable ceramic heating element that 1) reduces the risk of ceramic particle inhalation, 2) eliminates the use of potentially harmful adhesives and 3) dramatically lowers the amount of heavy metals used by over 80%.

Pictured above from left to right. Left: competitor 1 showing ceramic particles on testing surface. Right: competitor 2 showing ceramic particles on testing surface. Bottom: PurCore F1/R1 Ceramic technology showing no ceramic particles.
PurCore R1 has also undergone months of stress testing to ensure greater durability during manufacturing, ensuring that the risk of ceramic particles is greatly decreased during even strenuous manufacturing conditions.
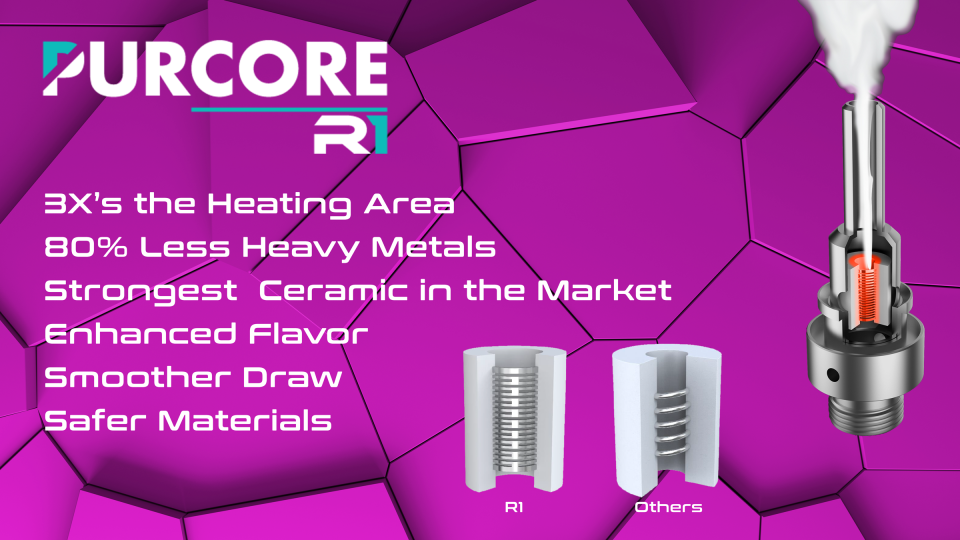
PurCore R1 has significant benefits when it comes to user experience. In over 200 use cases, the company's reporting shows that the R1 coil delivers superior flavor pass-through, meaning the oil tastes as it smells, greatly reducing degradation of flavor or burnt heating elements. The findings also show a substantial improvement in smoothness. The reason that these studies show this data is due to 1) the more advanced PCB (printed circuit board) which is the brain that controls smoking temperature, and 2) the use of 316L Stainless Steel which when combined together allows for more consistent and reliable heating temperatures. As shown below, you can see the traditional ceramic cell product used by nearly all of the company's competitors and the PurCore R1 where the R1 has a mesh coil design that consistently heats the entire surface of the ceramic without hot spots. This not only ensures better flavor, but a smoother draw with more consistent temperature control. The PurCore R1 delivers 3X's the heating area versus the largest competitors to PurTec.

The PurCore R1 coil is constructed with 316L stainless steel which is widely used in medical applications and compliant with FDA, RoHS 2.0, REACH. PurCore R1 contains 80% less heavy metals than the company's largest competitors and eliminates the need for adhesives. The R1 tested at an average vaporizer performance lifespan 5X's that of other competitive products, increasing the endurance of the product while delivering excellent flavor stability from the first puff to the last without dry or burnt wicking.
"The release of PurCore R1 in joint development with our strategic partner JWEI Group, is another major advancement in electronic vaporizer technology. Earlier this week we announced the PurCore Technology Platform, starting with the F1 technology which is the first cotton-free vape delivery system, and now the R1 technology which we believe will be the most heat stable and superior quality ceramic system, to be launched in Q1 2021. This technology has a primary application in 510-thread carts and testing has shown it to have substantial differentiating factors that will ultimately affect the user experience. With the JWEI Group Advanced Technology Research Institute, we have conducted thorough research and testing along with expansive consumer testing over the last year and the results have been profound. I strongly feel that this technology for 510 cartridges will become the new standard. The industry has relied on the same technology for the last four years, and has not developed anything we see as a substantial improvement in consumer safety or user experience," said Corey Mangold, CEO of Orchid Ventures. "Our mission continues to be the ultimate focus on engineering and design that will increase consumer safety and confidence. The level of testing that the regulatory bodies in the US require isn't sufficient to protect consumers. It's up to us as an industry to use the highest quality and safe materials, develop technology that challenges the status quo of vape safety, and ultimately deliver products that are free from contaminants, adhesives, harmful heavy metals, ceramic particles, and other known safety concerns. We believe that PurCore R1 is a breakthrough on all levels."
Dr. Bo Jiang, PhD. of the JWEI Group stated, "PurCore R1 was developed jointly with our JWEI Advance Technology Research Institute in Switzerland as another advancement of the µKera Technology Platform. We developed this technology to disrupt the 510 cartridge market and deploy a line of products that we feel, through over a year of substantial standardized testing, are the safest vape products available in the cannabis and CBD industry. As a leader in the space, we will continue to innovate and collaborate with our strategic partner PurTec, to launch the entire µKera line of products and technologies that will help prepare the industry for the potential future involvement of the FDA in the cannabis industry."
Please forward any questions regarding our new PurCore F1 vaporizer technology or inquiries about product availability to sales@purtecdesigns.com.
Or, learn more at www.PurTecDesigns.com
The Company announces that it has entered into a $100,000 line of credit facility with Augustiner Capital Ventures, LLC, a Delaware LLC ("Augustiner"). The line of credit facility is secured against the assets of the Company and will allow the Company to borrow up to a maximum of $100,000 at an interest rate of 10% per annum. Advances made under the line of credit will bear a 60-day term from the date each advance is made. In consideration for the line of credit facility, the Company has agreed to issue Augustiner warrants equal to 10 times the amount of each advance, to a maximum of 1,000,000 warrants. Each warrant will be exercisable into one common share of the Company for a period of one year from the date of issuance as such price that is equal to the greater of (i) the closing price of the Company's common shares on the date of the advance; and (ii) $0.05.
The Company has drawn a total of $99,999.90 from the line of credit facility and as such, has issued Augustiner a total of 999,999 warrants. Each warrant is exercisable at a price of CAD$0.06 per share until December 17, 2021.
ABOUT ORCHID VENTURES
Orchid Ventures is an Irvine, CA-based cannabis innovation company, that launched in Oregon and California in August 2017 and has since developed a mass-market brand and loyal consumer following with its premium cannabis products and unique vape hardware delivery system. Since July 2019, Orchid has diversified its efforts and has brought to market innovative services and product offerings to support brands throughout the global cannabis industry. Orchid has diversified its portfolio to include PurTec Delivery Systems, a company that produces, markets and sells clean vaporizer hardware that has been emissions tested against the most stringent standards in the world set forth by the EU and has unrivaled product quality and value pricing. Orchid, through its wholly-owned subsidiary, has launched a patented and clinically proven bioavailability solution to increase the absorption of THC and other cannabinoids making products much more effective and an activation time of less than ten minutes. With a continued focus on brand and intellectual property development, Orchid will continue to create new and innovative products and technologies, then bring them to the global cannabis marketplace and set the gold standard for delivery systems whether it's vape or formulation sciences. Orchid's management brings significant branding, product development and distribution experience with a proven track record of scaling businesses and building sustainable revenue growth through value-generating partnerships and innovation that creates enterprise value. Learn more at https://orchidessentials.com/
ON BEHALF OF THE BOARD OF DIRECTORS - ORCHID VENTURES, INC.
Corey Mangold
CEO and Director
investors@orchidessentials.com
Investor Relations
Corey Mangold
949-357-5818
corey@orchidessentials.com
The CSE does not accept responsibility for the adequacy or accuracy of this release.
Safe Harbor Statement
Except for historical information contained herein, statements in this release may be forward-looking and made pursuant to the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Words such as "anticipate", "believe", "estimate", "expect", "intend" and similar expressions, as they relate to Orchid Ventures, Inc. and Orchid Essentials any of its affiliates or subsidiaries (collectively, the "Company") or its management, identify forward-looking statements. These statements are based on current expectations, estimates and projections about the Company's business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may, and probably will, differ materially from what is expressed or forecasted in such forward-looking statements due to numerous factors, including those described above and those risks discussed from time to time in the Company's Canadian securities regulatory filings with sedar.com, Factors which could cause actual results to differ materially from these forward-looking statements include such factors as (i) the development and protection of our brands and other intellectual property, (ii) the need to raise capital to meet business requirements, (iii) significant fluctuations in marketing expenses, (iv) the ability to achieve and expand significant levels of revenues, or recognize net income, from the sale of our products and services, (v) the Company's ability to conduct the business if there are changes in laws, regulations, or government policies related to cannabis, (vi) management's ability to attract and maintain qualified personnel necessary for the development and commercialization of its planned products, and (vii) other information that may be detailed from time to time in the Company's Canadian securities regulatory filings with sedar.com. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
SOURCE: Orchid Ventures, Inc.
View source version on accesswire.com:
https://www.accesswire.com/621332/Orchid-Ventures-Subsidiary-PurTec-Announces-the-Launch-of-PurCore-R1-Mesh-Coil-Cartridge-Technology
