Pacira's (PCRX) Label Expansion Study on Exparel Meets Goal
Pacira BioSciences, Inc. PCRX announced positive top-line data from a phase III study evaluating its marketed drug, Exparel, as a single-dose sciatic nerve block in the popliteal fossa for postsurgical regional analgesia in patients undergoing bunionectomy.
Data from the study showed that treatment with Exparel led to a statistically significant reduction in cumulative pain scores from 0 to 96 hours as compared to bupivacaine HCl, the study’s primary endpoint.
Patients treated with Exparel also achieved statistical significance for the reduction in postsurgical opioid consumption and percentage of opioid-free subjects through 96 hours versus bupivacaine HCl, the study’s secondary endpoint.
Treatment with Exparel was generally well tolerated with a safety profile similar to bupivacaine HCl.
The above-mentioned study is Pacira’s second phase III study to evaluate Exparel for a new indication.
Shares of the company have lost 8.1% so far this year compared with the industry’s decline of 25.1%.
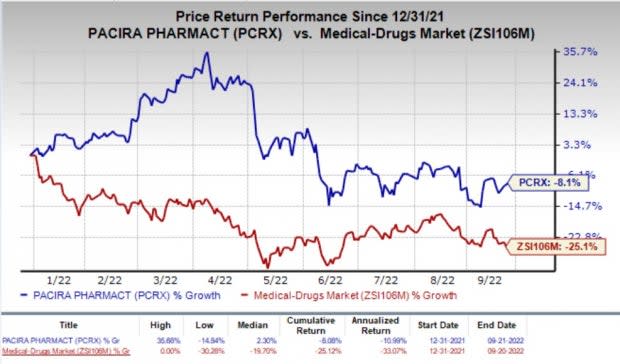
Image Source: Zacks Investment Research
Earlier this month, the company announced positive top-line data from a phase III study evaluating Exparel as a single-dose femoral nerve block in the adductor canal for postsurgical regional analgesia in patients undergoing total knee arthroplasty.
The study met its primary endpoint as well as the secondary endpoint.
PCRX plans to submit a supplemental new drug application (sNDA) to the FDA seeking label expansion of Exparel to include sciatic nerve blocks in the popliteal fossa as well as femoral nerve blocks in the adductor canal in early 2023.
Exparel, a liposome injection of bupivacaine, is indicated as a single-dose administration into the surgical site to produce postsurgical analgesia for patients in the United States.
In March 2021, the FDA approved Pacira’s supplemental new drug application seeking approval of Exparel for use in children aged six years and above.
Pacira’s flagship product, Exparel, has witnessed a strong uptake since its launch and has been a constant revenue driver thereafter. The drug generated sales worth $266.2 million in the first six months of 2022, reflecting an increase of almost 8.7% year over year.
Successful label expansion of Exparel into additional indications is likely to boost sales further in the days ahead.
Zacks Rank & Stocks to Consider
Pacira currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the same sector are Infinity Pharmaceuticals, Inc. INFI, Assertio Holdings, Inc. ASRT and Calithera Biosciences, Inc. CALA, all carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Infinity Pharmaceuticals’ loss per share estimates narrowed 11.5% for 2022 and 13.3% for 2023 in the past 60 days.
Earnings of Infinity Pharmaceuticals have surpassed estimates in each of the trailing four quarters. INFI delivered an earnings surprise of 7.14%, on average.
Assertio’s earnings per share estimates have increased 27.5% for 2022 and 13.8% for 2023 in the past 60 days.
Earnings of Assertio surpassed estimates in three of the trailing four quarters and missed on the other occasion. ASRT delivered an earnings surprise of 126.39%, on average.
Calithera Biosciences’ loss per share estimates narrowed 37.3% for 2022 and 39.8% for 2023 in the past 60 days.
Earnings of Calithera Biosciences surpassed estimates in three of the trailing four quarters and missed on the other occasion. CALA delivered an earnings surprise of 21.08%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Infinity Pharmaceuticals, Inc. (INFI) : Free Stock Analysis Report
Pacira BioSciences, Inc. (PCRX) : Free Stock Analysis Report
Calithera Biosciences, Inc. (CALA) : Free Stock Analysis Report
Assertio Holdings, Inc. (ASRT) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
