Thermo Fisher (TMO) Gets FDA PMA for Oncomine Dx Target Test
Thermo Fisher Scientific Inc. TMO recently announced the receipt of the FDA premarket approval (PMA) for its Oncomine Dx Target Test as a companion diagnostic (CDx) to help identify epidermal growth factor receptor (EGFR) Exon20 insertion mutations in patients with non–small cell lung cancer who may undergo treatment with RYBREVANT. This is the second approval for Oncomine Dx Target Test as a CDx for EGFR Exon20 insertion mutant patients and the 12th NSCLC approval globally.
It is worth to be mentioned that lung cancer is the main cause of cancer deaths worldwide. EGFR Exon20 insertion mutations, specifically, are linked with resistance to immune checkpoint inhibitor therapies and poor patient prognosis and are generally under-detected by conventional, single-gene testing methods. This is fuelling the need for more comprehensive biomarker testing with NGS technology.
The recent development is likely to boost Thermo Fisher’s Specialty Diagnostics segment.
More on Oncomine Dx Target Test
The Oncomine Dx Target Test evaluates 23 genes associated with NSCLC simultaneously. The Oncomine Dx Target Test is the first and only FDA-approved next-generation sequencing (NGS) CDx on formalin-fixed, paraffin-embedded (FFPE) tissue for checking RYBREVANT eligibility in patients whose disease has evolved on or after platinum-based chemotherapy.
Significance of the Oncomine Dx Target Test
Per Thermo Fisher’s management, the FDA's approval of Oncomine Dx Target Test will allow clinicians to use FFPE tissue samples to spot patients in the United States who may benefit from the important new therapy.
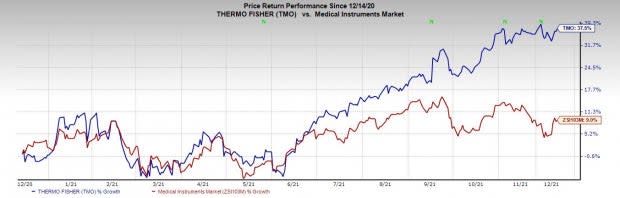
Image Source: Zacks Investment Research
Thermo Fisher also noted that NGS technology is vital to make connections and advance precision medicine in situations where conventional testing may overlook key mutations that could match patients with targeted therapies. Moreover, Thermo Fisher is looking forward to expanding the registration of the Oncomine Dx Target Test as a companion diagnostic for RYBREVANT across the globe to help enhance outcomes for more patients.
Industry Prospects
Per Fortune Business Insights report, the global lung cancer screening market is projected to rise from $2.80 billion in 2021 to $4.85 billion in 2028, at a CAGR of 8.1%.
Rising incidence of lung cancer, increasing awareness levels about the symptoms and the seriousness of the condition, technological advancements and increased focus on the development of lung cancer-specific biomarkers are the factors driving the market.
Recent Developments
In December 2021, Thermo Fisher announced a significant expansion of the K-12 ReadyCheckGo COVID-19 testing solution in Texas in collaboration with Color Health. ReadyCheckGo allows schools to test students quickly, manage the logistics of monitoring results, and update parents and staff accordingly.
In November 2021, Thermo Fisher announced the launch of a new in vitro diagnostic (IVD) system that will enable the company to expand its assay menus and IVD testing capabilities going forward. The Applied Biosystems QuantStudio 7 Pro Dx Real-Time PCR System is a compact instrument that effortlessly transitions from development to validation for maximum productivity.
Price Performance
Shares of the company have gained 37.5% in a year’s time compared with the industry’s rise of 9%.
Zacks Rank and Other Key Picks
Thermo Fisher currently carries a Zacks Rank #2 (Buy).
A few other top-ranked stocks from the broader medical space are Chemed Corporation CHE, Laboratory Corporation of America Holdings, or LabCorp LH and Medpace Holdings, Inc. MEDP.
Chemed has a long-term earnings growth rate of 7.7%. The company surpassed earnings estimates in three of the trailing four quarters and missed in one, delivering a surprise of 5.6%, on average. It currently carries a Zacks Rank #2.
Chemed has outperformed its industry over the past year. CHE has gained 3.7% against a 35.6% industry decline.
LabCorp reported third-quarter 2021 adjusted earnings per share (EPS) of $6.82, which surpassed the Zacks Consensus Estimate by 42.9%. Revenues of $4.06 billion outpaced the Zacks Consensus Estimate by 13.4%. It currently carries a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
LabCorp has an estimated long-term growth rate of 10.6%. LH surpassed estimates in the trailing four quarters, the average surprise being 25.7%.
Medpace reported third-quarter 2021 adjusted EPS of $1.29, surpassing the Zacks Consensus Estimate by 20.6%. Revenues of $295.57 million beat the Zacks Consensus Estimate by 1.2%. It currently carries a Zacks Rank #1.
Medpace has an estimated long-term growth rate of 16.4%. MEDP surpassed estimates in the trailing four quarters, the average surprise being 11.9%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Laboratory Corporation of America Holdings (LH) : Free Stock Analysis Report
Thermo Fisher Scientific Inc. (TMO) : Free Stock Analysis Report
Chemed Corporation (CHE) : Free Stock Analysis Report
Medpace Holdings, Inc. (MEDP) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
