Ultragenyx (RARE) Acquires Rights of AAV Gene Therapy Candidate
Ultragenyx Pharmaceutical Inc. RARE announced that it has entered into an exclusive license agreement with New York-based biopharmaceutical company, Abeona Therapeutics Inc. ABEO, wherein it will acquire global rights of the latter’s gene therapy candidate ABO-102 (now UX111).
The AAV gene therapy candidate, UX111, is currently being evaluated in the open-label, global phase I/II Transpher A study for the treatment of patients with Sanfilippo syndrome type A (MPS IIIA), a rare, fatal lysosomal storage disease. Currently, there is no approved therapy for the given indication.
Per the latest agreement, Ultragenyx will undertake the responsibility of the ABO-102 program while ABEO will be entitled to receive tiered royalties of up to 10% on net sales and milestone payments upon potential approval of the product.
Shares of Ultragenyx have declined 35.3% so far this year compared with the industry’s decrease of 24.9%.
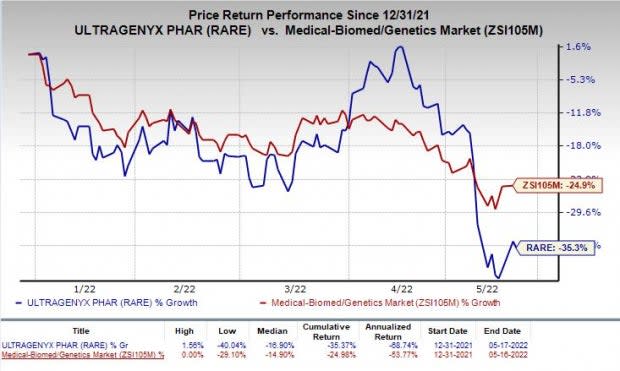
Image Source: Zacks Investment Research
The phase I/II Transpher A study is investigating ABO-102 for treating patients with MPS IIIA. The primary endpoints of the study are neurodevelopment and safety.
Per the press release, Abeona has completed a Type B meeting, which was a success, with the FDA regarding the Transpher A study. The findings from the study are likely to support regulatory filing and approval of ABO-102 for MPS IIIA.
Till date, data from the study have shown that treatment with a single intravenous dose of ABO-102 has the potential to offer children with MPS IIIA sustained neurocognitive development when treated at early stages of their disease.
If successfully developed and upon potential approval, ABO-102 might serve an area of highly unmet medical need and offer a transformative therapy for children with MPS IIIA.
The FDA has already granted Regenerative Medicine Advanced Therapy, Fast Track, Rare Pediatric Disease and Orphan Drug designations to ABO-102 for the treatment of MPS IIIA.
The above deal looks a good strategic fit for Ultragenyx, given that it already has some gene therapy candidates in its pipeline that are being developed for various indications.
Zacks Rank & Stocks to Consider
Ultragenyx currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector are Applied Therapeutics, Inc. APLT and AegleaBio Therapeutics, Inc. AGLE, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Applied Therapeutics’ loss per share estimates have narrowed 27.1% for 2022 and 20.2% for 2023 over the past 60 days.
Earnings of Applied Therapeutics have surpassed the Zacks Consensus Estimate in one of the trailing four quarters, met the same once and missed the same on the other two occasions. APLT delivered an earnings surprise of -0.67% on average.
AegleaBio Therapeutics’ loss per share estimates have narrowed 23.2% for 2022 and 30.6% for 2023 over the past 60 days.
Earnings of AegleaBio Therapeutics have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions. AGLE delivered an earnings surprise of 9.47% on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ultragenyx Pharmaceutical Inc. (RARE) : Free Stock Analysis Report
Abeona Therapeutics Inc. (ABEO) : Free Stock Analysis Report
Aeglea BioTherapeutics, Inc. (AGLE) : Free Stock Analysis Report
Applied Therapeutics Inc. (APLT) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
