Vaxart Gets New CEO, Focuses on Coronavirus Vaccine Program
Vaxart, Inc. VXRT announced that it has appointed Andrei Floroiu as the company’s new chief executive officer (CEO) with immediate effect. He will succeed Dr. Wouter Latour, the current CEO.
Meanwhile, Dr. Latour will remain as the chairman on the company’s board, having led it for the last eight years.
On a positive note, Floroiu has vast experience to count on. He is a veteran who helmed various organizations over the years. Most recently, he held executive positions at Agenus, Inc wherein he forged various strategic partnerships and effected other transactions. He has also been Vaxart's board member since April 2020 and will continue to be one of its directors while assuming his new role.
The CEO reshuffle at Vaxart happens when the company initiated a program in January 2020 to develop vaccine candidates for fighting the novel coronavirus infection. The new vaccine candidate will be based on the company’s proprietary oral vaccine platform, VAAST.
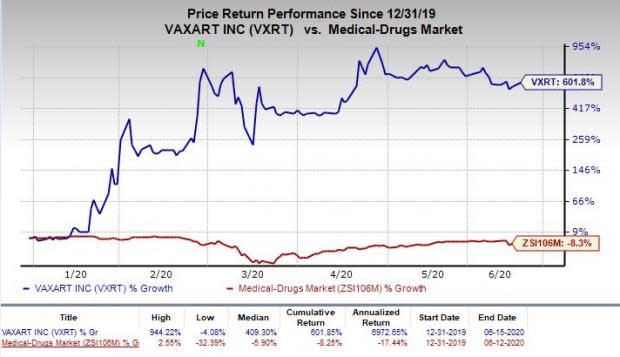
Shares of Vaxart were up 5.5% following the above announcement on Monday. In fact, so far this year, the stock has skyrocketed 601.8% versus the industry’s decline of 8.3%.
In April this year, the company announced favorable data from pre-clinical studies on its oral vaccine candidates against SARS CoV-2, the virus that causes COVID-19. Several vaccine candidates were able to generate immune responses after a single dose in all the animals under the studies was tested.
The company is looking to develop a vaccine candidate that can generate mucosal immune responses in addition to serum antibody responses, which are potentially significant for protection against the deadly COVID-19 infection.
Notably, last month, Vaxart selected its lead COVID-19 vaccine candidate and signed a contract with KindredBio to manufacture vaccines in bulk under cGMP to complement the manufacturing capacity of its partner Emergent BioSolutions EBS.
Vaxart has a manufacturing agreement with Emergent BioSolutions wherein the latter will produce the clinical material for the company’s experimental oral vaccine candidates against COVID-19.
As a matter of fact, to address the global pandemic issue, several large and smaller pharma/biotech companies are racing against time to successfully develop a treatment or a vaccine.
Many companies commenced a phase I study on their respective coronavirus vaccine candidates. Small biotech Novavax, Inc. NVAX is evaluating its coronavirus vaccine candidate, NVX-CoV2373, in a phase I/II study. Preliminary immunogenicity and safety data from the same is expected in July.
Another biotech Moderna, Inc. MRNA will soon begin a phase III study on mRNA-1273, its vaccine candidate against COVID-19.
Zacks Rank
Vaxart currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +24.1% per year.
These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
Emergent Biosolutions Inc. (EBS) : Free Stock Analysis Report
VAXART, INC. (VXRT) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
