SRNE: Sorrento Reports Positive Initial PK Data From TRIBECA Study
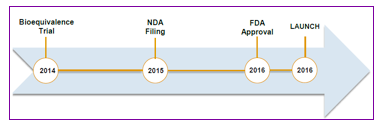
By Grant Zeng, CFA Earlier today (Oct. 14, 2014), Sorrento Therapeutics (SRNE) announced positive results from recently analyzed pharmacokinetic (PK) data from the first eight (8) patients enrolled in its ongoing pivotal TRIBECA™ registrational trial. The Original Design of Pivotal Trial of Cynviloq In March, 2014, Sorrento initiated the pivotal clinical trial of Cynviloq for the treatment of metastatic breast cancer and non-small cell lung cancer. The registration trial is referred to as TRIBECA™ (TRIal designed to evaluate BioEquivalence between Cynviloq™ and Abraxane®), which is an open-label, randomized, multi-center, single-dose, crossover registration study being conducted at clinical sites across the U.S., EU, and Singapore. Based on the End-of-Phase II meeting with the FDA in July 2013, this trial was designed to gain marketing approval for Cynviloq under the 505(b)(2) regulatory pathway in the U.S. using Abraxane as the reference drug. The 505(b)(2) BE trial design will enroll 100 patients with MBC and include a crossover 2 cycle design. The first cycle of the trial will involve 50 patients in the Abraxane® arm and 50 patients in the Cynviloq arm. The second cycle of the trial will crossover the patients into the opposite treatment groups. Dosage for both drugs will be 260 mg/m2 with 30 minutes infusion. The endpoints will be AUC and Cmax (90% CI). The BE trial will take about 12 months to complete, which will consume roughly $5 million in direct cost. The Positive Initial PK Data The following chart illustrates the initial data from the first 8 patients. The almost coincidental curve between Cynviloq and Abraxane indicates that Cynviloq and Abraxane have similar pharmacokinetics in the patients. These data support earlier completion of the study. The Modified Trial Protocol Sorrento amended the current BE cross-over design protocol of the TRIBECA study to un-blind the first 8 patients to reassess the sample size of 100 patients estimated from simulation of historical PK data. The Company does not plan to un-blind additional patient data. Current sample size point estimates suggest that the enrollment target for the current study can be reduced to nearly half of the original target. Based on the initial positive data, Sorrento plans to reduce the TRIBECA patient sample size to 53 patients to accelerate filing for FDA approval of Cynviloq Sorrento expects to file a New Drug Application with the FDA in the first half of 2015. According to this schedule, we expect the FDA approval and launch of Cynviloq in 2H2016. Sorrento Out-licenses Anti-PD-L1 antibody to China Oncology Focus On Oct. 3, 2014, Sorrento Therapeutics (SRNE) announced that China Oncology Focus Limited has licensed Sorrento's fully human immune-oncology anti-PD-L1 monoclonal antibody (mAb) STI-A1014. China Oncology Focus is an Affiliate of Lee's Pharmaceutical Holdings Limited, a public biopharmaceutical company based in Hong Kong with operations in China. As part of the deal, Lee's Pharma will have exclusive rights to develop and commercialize the antibody for the greater Chinese market, including Mainland China, Hong Kong, Macau, and Taiwan. In turn, Sorrento will receive an up-front payment, potential future milestone payments and high single digit to double digit royalties on future net sales up to $46 million. In addition, Lee's Pharma will invest $3.6 million by purchasing common stock in Sorrento at a substantial premium to the current market price. STI-A1014 was discovered using Sorrento's proprietary G-MAB® library platform, which has the potential to generate a variety of unique antibodies for internal development or for partnerships. Lee’s Pharma plans to start a Phase I clinical trial of the anti-PD-L1 antibody in China in 2015. Despite the rapid growth of mAbs, there are still considerable unmet medical needs. One area is the development of antibodies targeting immune checkpoints — immunotherapies for cancer and other indications. Both CTLA-4 and PD-1/PDL-1 inhibitors have shown impressive results in clinical care and in ongoing clinical studies. Recent approval of Yervoy (CTLA-4 antibody from BMS) and Keytruda (PD-1 antibody from Merck) demonstrated the importance of immune checkpoint inhibitors in the treatment of cancer. We think the cancer immunotherapy by immune checkpoint inhibitors will enter widespread clinical use and will command a large market share of the cancer care market within the next decade. We welcome this partnership and think it’s positive to the Sorrento. The partnership not only boosts the Company’s balance sheet in a non-dilutive way, but also validates its antibody technology. READ THE LATEST FULL RESEARCH REPORT HERE SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR and to view our disclaimer.