AbbVie Gets FDA Nod for Venclexta Label Expansion in Leukemia
AbbVie, Inc. ABBV announced that the FDA has approved the label expansion of its cancer drug Venclexta and Roche’s RHHBY Rituxan to include minimal residual disease (“MRD”)-negativity data from phase III MURANO study.
In case of MRD-negativity, chronic lymphocytic leukemia (“CLL”) cells fall below the ratio of 1:10000 lymphocytes in the blood or bone marrow of patients, making the disease undetectable during normal diagnosis.
The study evaluated the combination regimen in relapse/refractory CLL patients who have received at least one prior therapy compared to Teva Pharmaceutical’s TEVA Treanda (bendamustine) plus Rituxan.
Data from the study showed that more than half of patients (53%) in Venclexta+Rituxan -arm achieved MRD-negativity (undetectable disease) compared to 12% of the patients in Treanda-arm. The MRD-negativity was achieved after approximately nine months on Venclexta+Rituxan therapy.
Notably, in June, the combination therapy was granted approval for the treatment of patients with relapsed/refractory CLL or small lymphocytic lymphoma (SLL), with or without 17p deletion, who have received at least one prior therapy. The approval was based on lower risk of disease progression and better overall response rate data compared to Treanda combination in the MURANO study.
Venclexta in combination with Rituxan is the first chemotherapy-free combination therapy, which has shown a fixed treatment duration for previously-treated CLL patients in clinical study. Treatment can be stopped after a duration of approximately two years.
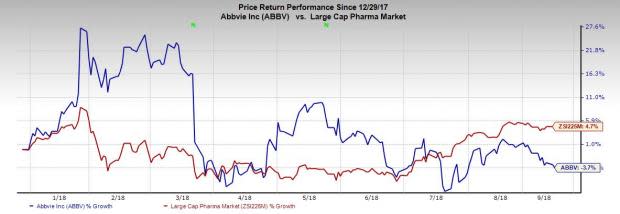
AbbVie’s shares have lost 3.7% this year so far against the industry’s gain of 4.7%.
The company states that MRD-negativity is becoming an increasingly important criteria in the treatment of rare blood cancer, CLL, in previously-treated patients. Thus, addition of this data to the drug’s label may help physicians to prescribe it to a larger patient base. Per the press release, 20,000 new patients are diagnosed with CLL every year in the United States.
Also, in July, AbbVie filed a regulatory application in the United States for Venclexta in acute myeloid leukemia ("AML") while a phase III program in multiple myeloma is also progressing well.
AbbVie Inc. Price
AbbVie Inc. Price | AbbVie Inc. Quote
Zacks Rank & Stock to Consider
AbbVie currently carries a Zacks Rank #3 (Hold). A better-ranked large-cap pharma stock is Eli Lilly & Company LLY, with a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Lilly’s earnings estimates increased 6% for 2018 and 3.6% for 2019 over the past 60 days. The company delivered a positive earnings surprise in all the trailing four quarters, with an average beat of 10.15%. Shares of Lilly have gained 25.1% this year so far.
Wall Street’s Next Amazon
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Click for details >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Teva Pharmaceutical Industries Ltd. (TEVA) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

