CureVac (CVAC) Begins Influenza Shot Dosing in Phase I Study
CureVac CVAC announced that it has initiated a phase I study that is evaluating CVSQIV, its second-generation multivalent vaccine candidate, for seasonal influenza. The vaccine is developed in collaboration with GlaxoSmithKline GSK.
CVSQIV is an investigational mRNA-based prophylactic vaccine that combines multiple non-chemically modified mRNA constructs in a single vaccine designed to induce an immune response against the relevant targets of four different influenza strains.
CureVac has designed the phase I study to evaluate the safety, reactogenicity and immunogenicity of CVSQIV in up to 240 healthy participants of two age groups, one between 18 and 55 and another aged 65 years and above.
CureVac’s shares have plunged 84.5% in the trailing 12 months in comparison with the industry’s 39.9% decline.
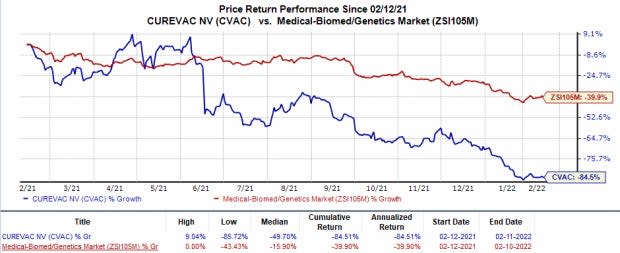
Image Source: Zacks Investment Research
Both CureVac and Glaxo are also planning to start a clinical study to test the use of chemically modified mRNA for influenza in 2022.
Please note that the influenza vaccine candidate is part of the strategic collaboration between CureVac and GlaxoSmithKline. In July 2020, both CureVac and Glaxo entered into a strategic collaboration to develop mRNA-based products for a broad infectious disease vaccine program. As part of this deal, Glaxo also acquired an equity stake in CureVac.
The collaboration between CureVac and Glaxo was further extended to develop a second-generation COVID-19 vaccine, CV2CoV, which is expected to enter into clinical studies later this year.
Moderna MRNA is evaluating mRNA-1010, its quadrivalent mRNA-based vaccine candidate, against seasonal influenza. Last December, Moderna announced interim data from the phase I study, which demonstrated that immunization with mRNA-1010 boosted immune response against all four strains of flu — influenza A/H1N1, A/H3N2 and influenza B/Yamagata- and B/Victoria-lineages — tested in both younger and older adults. While Moderna has already completed enrolment in the phase II study of mRNA-1010, preparations for a phase III study are currently ongoing.
CureVac N.V. Price

CureVac N.V. price | CureVac N.V. Quote
Zacks Rank & Key Pick
CureVac currently carries a Zacks Rank #4 (Sell). A better-ranked stock in the same sector is Vertex Pharmaceuticals VRTX, which presently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Vertex Pharmaceuticals’ earnings per share estimates for 2022 have increased from $13.39 to $14.33 in the past 30 days. The same for 2023 has risen from $14.10 to $15.31 in the past 30 days. VRTX has risen 12.1% in the past year.
Earnings of Vertex Pharmaceuticals beat estimates in each of the last four quarters, delivering a surprise of 10%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
CureVac N.V. (CVAC) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
