Pfizer's (PFE) Cibinqo Gets FDA Nod for Atopic Dermatitis
Pfizer Inc. PFE announced that the FDA has approved its oral, once-daily, JAK1 inhibitor, Cibinqo (abrocitinib), for the treatment of refractory moderate-to-severe atopic dermatitis (AD) — also called eczema — in adult patients who did not respond to treatment with other drugs or when the use of other treatments is not recommended. Cibinqo is already approved in Europe, U.K. and Japan.
Cibinqo has been approved at two dose levels for the given indication - 100 mg and 200 mg. The 200 mg dose is to be prescribed when patients do not respond to treatment with 100 mg dose. The FDA has also approved a third dose level of 50 mg to treat AD patients specifically with moderate renal impairment (kidney failure), patients receiving treatment with inhibitors of cytochrome P450 (CYP) 2C19 or patients who have a poor metabolism of CYP2C19.
The FDA approval for Cibinqo is based on data from five clinical studies that evaluated Cibinqo in more than 1,600 patients. One of the studies also compared the drug with Sanofi SNY and Regeneron’s REGN blockbuster AD drug, Dupixent (dupilumab). Data from all these studies demonstrated that treatment with Cibinqo improved disease severity, itch and skin clearance.
Shares of Pfizer have surged 49.7% in the trailing 12 months compared with the industry’s 14.7% rise.
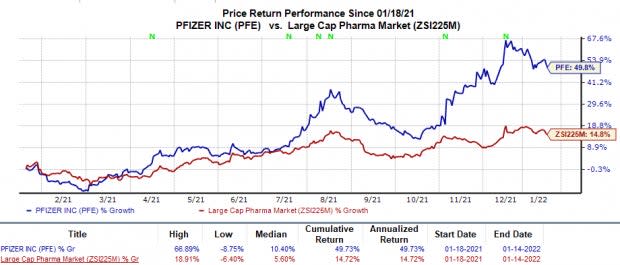
Image Source: Zacks Investment Research
We remind investors that the approval for Cibinqo in AD comes after the FDA extended the review period of the regulatory application seeking approval for abrocitinib twice in the last year.
This delay was due to the FDA’s review of the final data from the post-marketing safety study of Pfizer’s another JAK inhibitor, Xeljanz, in rheumatoid arthritis (RA). The FDA’s review concluded that patients treated with both low and high doses of Xeljanz experienced a higher rate of serious heart-related events such as heart attack, stroke, cancer, blood clots, and death compared to those treated with tumor necrosis factor (TNF) inhibitors. Hence, the FDA limited the use of Xeljanz to certain patients who have not responded to or cannot tolerate one or more TNF blockers and issued a directive last September to add warnings about these increased risks on Xeljanz’s label
This FDA directive was also extended to the labels of other JAK inhibitors medications, AbbVie’s ABBV Rinvoq and Eli Lilly’s Olumiant, both of which are approved for RA indication.
Last week, the FDA also granted label expansion to AbbVie’s Rinvoq to treat moderate-to-severe AD in adults and children aged 12 years and above who did not respond to treatment with other drugs or when the use of other treatments is not recommended.
Last month, AbbVie received FDA approval for Rinvoq in active psoriatic arthritis indication.
We note that dupilumab is a fully human monoclonal antibody developed by Regeneron in partnership with Sanofi as part of a global collaboration agreement. Both Cibinqo and Rinvoq face stiff competition from Regeneron/Sanofi’s Dupixent, which has already been approved by the FDA to treat moderate-to-severe atopic dermatitis in patients aged six years and older.
Pfizer Inc. Price
Pfizer Inc. price | Pfizer Inc. Quote
Zacks Rank
Pfizer carries a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
