Acadia (ACAD) Expands Existing Licensing Deal With Neuren
Acadia Pharmaceuticals Inc. ACAD announced that it is expanding its current licensing agreement for trofinetide with Neuren Pharmaceuticals. Under the agreement, ACAD has now acquired rights to market trofinetide outside North America along with exclusive global rights to Neuren’s development candidate, NNZ-2591, in Rett syndrome and Fragile X syndrome.
In March 2023, trofinetide received FDA approval for the treatment of Rett syndrome in adult and pediatric patients two years of age and older. The drug was launched in the United States in April under the brand name, Daybue. Per Acadia, Daybue currently stands as the first and only drug approved for the treatment of Rett syndrome.
The stock of the company climbed about 23% in the after-market hours on Thursday, following the positive news. Year to date, shares of Acadia have shot up 62.1% against the industry’s 10% decline.
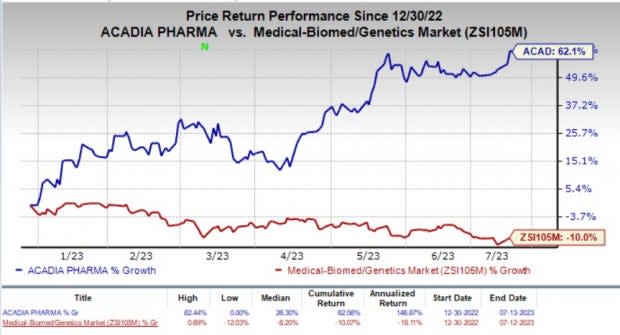
Image Source: Zacks Investment Research
NNZ-2591, Neuren’s investigational candidate, is currently under development in four other rare neurodevelopmental syndromes, barringRett syndrome and Fragile X syndrome. By acquiring global rights for this candidate, ACAD is looking to further advance the global potential of its current development portfolio.
Per the terms of the licensing agreement, Neuren is entitled to receive an upfront payment of $100 million from Acadia. Neuren is also eligible to receive royalty payments and milestone payments from ACAD separately, upon the achievement of certain pre-specified revenue milestones. Additionally, Neuren will receive tiered royalty payments from Acadia which will range from the mid-teens to low-twenties percent of trofinetide net sales outside of North America.
In North America, the arrangement for all royalties and milestone payments for trofinetide remains unchanged from the existing North American license agreement between Acadia and Neuren. ACAD also stated that all future payments to Neuren, related to the successful development and approval of NNZ-2591 in Rett syndrome and Fragile X syndrome, will be identical to the payments for trofinetide in both inside and outside North America.
Rett syndrome is a rare, complex genetic neurodevelopmentaldisorder that may occur over four stages, affecting approximately 6,000-9,000 patients in the United States annually. Currently, approximately 4,500 patients are diagnosed with Rett syndrome according to an analysis of healthcare claims data. Rett syndrome is characterized by progressive loss of motor skills and language and it primarily affects females.
In the same press release, the company also reported its preliminary net sales of $21 million to $23 million for Daybue in the second quarter of 2023. On the other hand, Nuplazid’s preliminary net sales for the second quarter of 2023 is expected between $140 million and $144 million.
For the full year, Acadia expects to generate revenues in the range of $140-$144 million from the sales of Nuplazid. For Daybue, the company expects total sales in the band of $45-$55 million in the third quarter of 2023.
ACADIA Pharmaceuticals Inc. Price and Consensus
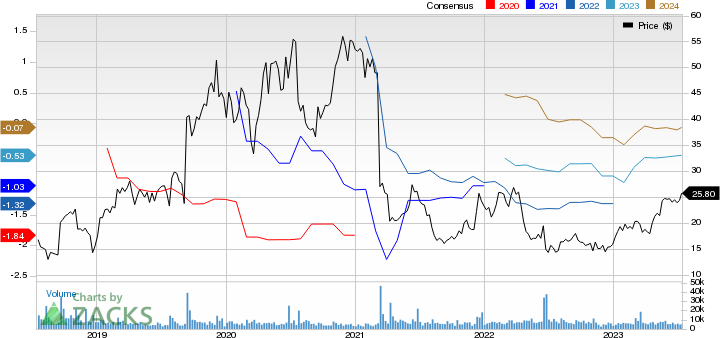
ACADIA Pharmaceuticals Inc. price-consensus-chart | ACADIA Pharmaceuticals Inc. Quote
Zacks Rank and Stocks to Consider
Acadia currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the same industry are ADC Therapeutics ADCT, Anixa Biosciences ANIX and Akero Therapeutics AKRO, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for ADC Therapeutics’ 2023 loss per share has widened from $2.58 to $2.61. During the same period, the estimate for ADC Therapeutics’ 2024 loss per share narrowed from $2.72 to $2.45. Year to date, shares of ADCT have lost 59.7%.
ADCT beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 10.70%.
In the past 90 days, the Zacks Consensus Estimate for Anixa Biosciences’ 2023 loss per share has narrowed from 43 cents to 39 cents. During the same period, the estimate for Anixa Biosciences’ 2024 loss per share has narrowed from 46 cents to 38 cents. Year to date, shares of ANIX have lost 25.2%.
ANIX beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 31.21%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $2.97 to $2.80. During the same period, the estimate for AKRO’s 2024 loss per share narrowed from $3.40 to $3.27. Year to date, shares of AKRO have lost 17.3%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ADC Therapeutics SA (ADCT) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
ANIXA BIOSCIENCES INC (ANIX) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
