Acadia (ACAD) Q2 Earnings & Revenues Beat Estimates, '23 View Up
Acadia Pharmaceuticals Inc. ACAD reported second-quarter 2023 earnings of 1 cent per share. The Zacks Consensus Estimate was pegged at a loss of 12 cents per share. In the year-ago quarter, the company had incurred a loss of 21 cents per share.
The company recorded net product revenues of $165.2 million in the reported quarter, surpassing the Zacks Consensus Estimate of $154 million. Acadia’s net product revenues comprised of revenues from two of its marketed products, such as Nuplazid (pimavanserin) and the newly-approved Daybue (trofinetide).
Nuplazid is an FDA-approved treatment for hallucinations and delusions associated with Parkinson’s disease psychosis. On the other hand, Acadia’s second product, Daybue, was approved by the FDA in March 2023 for the treatment of Rett syndrome in adult and pediatric patients two years of age and older.
Daybue was made available in the U.S. market in April 2023.
Year to date, shares of Acadia have shot up 83.4% against the industry’s 12.3% fall.

Image Source: Zacks Investment Research
Quarter in Detail
In the reported quarter, revenues from Nuplazid sales increased 5.5% year over year to $142 million. Nuplazid sales increased 19.8% sequentially in the second quarter. Per the company, the uptick in Nuplazid sales was primarily driven by an increase in volume due to demand from new patient starts of Nuplazid and a higher average net selling price. The reported second-quarter figure beat our model estimate of $132.9 million.
In the first quarter of commercialization in April 2023, Daybue recorded net product sales of $23.2 million for the reported quarter. Management claims that more than 400 prescribers have written prescriptions for Daybue, to date.
Research and development (R&D) expenses in the quarter were $58.8 million, down 22.2% year over year, owing to decreased costs in the prior year associated with pre-approval manufacturing supply expenses for trofinetide.
Selling, general and administrative (SG&A) expenses were $96 million, up 6.8% year over year. The improvement in the expenses can be attributed to increased commercial costs associated with the Daybue launch, partially offset by efficiencies in Acadia’s commercial support of Nuplazid.
Acadia had cash, cash equivalents and investments worth $375.4 million as of Jun 30, 2023, compared with $402.9 million as of Mar 31, 2023. The decrease in cash balance is on account of the $40 million milestone payment to Neuren related to Daybue’s first commercial sale.
2023 Financial Guidance Updated
Acadia expects third-quarter 2023 Daybue sales in the range of $45-$55 million.
ACAD now expects Nuplazid net sales in the range of $530-$545 million (previously $520-$550 million), thus streamlining its expectations for the full-year 2023.
The company expects R&D expenses in the $335-$355 million range (previously $235-$255 million. This guidance includes the $100 million upfront payment to Neuren in July for the expanded licensing agreement.
SG&A expenses are expected in the band of $380-$400 million (previously $360-$380 million) in 2023. The anticipated rise in SG&A expenses is on account of higher operating costs as a result of favorable business performance, including employee retention costs as well as Daybue incentive compensation and investments in patient support services.
Pipeline Updates
Acadia is currently evaluating pimavanserin in the phase III ADVANCE-2 study for treating negative symptoms of schizophrenia. The company reported completing enrollment in the study during second-quarter 2023, expecting to announce top-line results from the same in the first quarter of 2024.
Acadia is also developing ACP-204 as a potential treatment for Alzheimer’s disease psychosis. The company completed phase I development of ACP-204, witnessing a favorable safety and tolerability profile. Following a meeting with the FDA during the reported quarter, ACAD aligned with the regulatory body on dosing and plans to initiate a phase II/III program in the fourth quarter of 2023.
During the second quarter, the company also announced adding a new phase III development candidate, ACP-101, to its rare disease portfolio. The investigational, intranasal formulation of ACP-101 is set to be evaluated for treating hyperphagia in Prader-Willi syndrome (PWS). Acadia reported recently meeting with the FDA, reaching alignment to further evaluate the 3.2 mg dose of ACP-101 in a pivotal phase III study, which is expected to be initiated in thefourth quarter of 2023. If the phase III study becomes successful, ACAD plans to submit a new drug application for treating hyperphagia in PWS to the FDA.
Other Updates
In July 2023, Acadia announced expanding its current licensing agreement for trofinetide with Neuren Pharmaceuticals. Under the agreement, ACAD has now acquired rights to market trofinetide outside North America along with exclusive global rights to Neuren’s development candidate, NNZ-2591, in Rett syndrome and Fragile X syndrome.
ACADIA Pharmaceuticals Inc. Price, Consensus and EPS Surprise
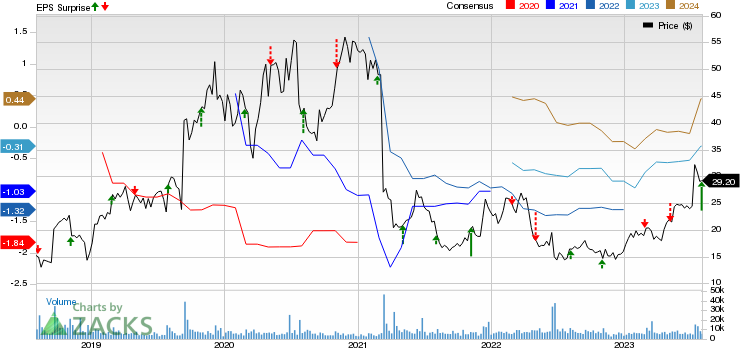
ACADIA Pharmaceuticals Inc. price-consensus-eps-surprise-chart | ACADIA Pharmaceuticals Inc. Quote
Zacks Rank and Other Stocks to Consider
Acadia currently has a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the overall medical sector, worth mentioning, are J&J JNJ, ADC Therapeutics ADCT and ADMA Biologics, Inc. ADMA, each carrying a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for J&J’s 2023 earnings per share has increased from $10.66 to $10.73. During the same period, the estimate for JNJ’s 2024 earnings per share has increased from $11.01 to $11.28. Year to date, shares of JNJ have lost 3.8%.
JNJ beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 5.58%.
In the past 90 days, the Zacks Consensus Estimate for ADC Therapeutics’ 2023 loss per share has widened from $2.60 to $2.61. During the same period, the estimate for ADC Therapeutics’ 2024 loss per share narrowed from $2.75 to $2.55. Year to date, shares of ADCT have lost 61.2%.
ADCT beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 10.70%.
In the past 90 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 14 cents to 8 cents. The consensus estimate for 2024 earnings is currently pegged at 7 cents per share. Year to date, shares of ADMA have gained 4.4%.
ADMA beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 19.13%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
ADC Therapeutics SA (ADCT) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
