ADC Therapeutics (ADCT) Dips on Halting Zynlonta Study
ADC Therapeutics’ADCT shares declined almost 22% on Jun 11 as it paused enrollment of new patients in a phase II study evaluating its only marketed product, Zynlonta, in previously untreated diffuse large B-cell lymphoma patients.
The decision was made after a careful review of aggregate data from the 40 patients already enrolled in the study, and consultation with the Data Monitoring Committee.
The review raised concerns about a potentially high number of respiratory-related events among the patients. It also revealed the death of seven patients and other five cases of serious respiratory problems during the study.
The mid-stage LOTIS-9 study evaluated expanded use of Zynlonta (loncastuximab tesirine-lpyl) in combination with Roche’s RHHBY rituximab for unfit or frail patients with previously untreated diffuse large B-cell lymphoma.
Zynlonta is presently approved by both FDA and EMA for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy.
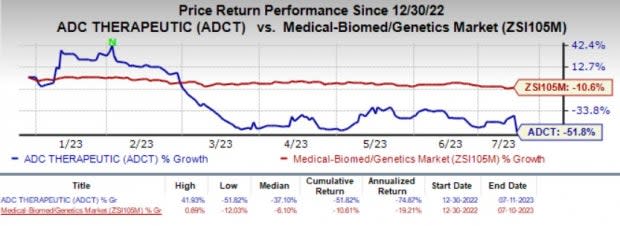
ADC Therapeutics’ shares have nosedived 51.8% year to date compared with the industry’s 10.6% decline.
Image Source: Zacks Investment Research
The 40 enrolled patients received the combination therapy. After receiving the therapy, 12 patients experienced respiratory-related adverse events. Per management, the investigations determined that 11 out of the 12 events, including six deaths, were not related to the Zynlonta treatment.
The company noted that all of the patients who passed away were at least 80 years old and had underlying health conditions, such as obstructive pulmonary disease, pulmonary edema, chronic bronchiectasis, idiopathic pulmonary fibrosis, or COVID-19 infection.
The company has informed all study investigators and regulatory authorities, including the FDA and the European Medicines Agency, about its decision to halt enrollment. ADC Therapeutics does not anticipate releasing additional data from the study by the end of the year.
In light of its decision, ADCT plans to conduct a comprehensive review of the implications for the Zynlonta program.
ADC Therapeutics SA Price and Consensus
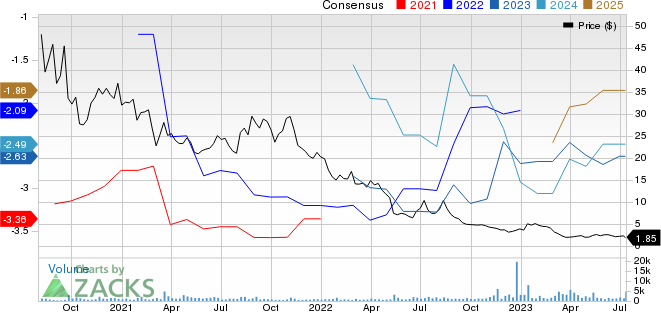
ADC Therapeutics SA price-consensus-chart | ADC Therapeutics SA Quote
Zacks Rank & Other Stocks to Consider
ADC Therapeutics currently carries a Zacks Rank #2 (Buy).
A couple of other top-ranked stocks in the overall healthcare sector are Akero Therapeutics AKRO and Omega Therapeutics OMGA, both carrying a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Zacks Consensus Estimate for Akero Therapeutics has narrowed from a loss of $2.92 per share to a loss of $2.80 for 2023 in the past 90 days. The company's shares have nosedived 18.2% year to date.
AKRO’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 19.13%.
The consensus mark for Omega Therapeutics has narrowed from a loss of $2.49 per share to a loss of $2.05 for 2023 in the past 90 days. Shares of the company have nosedived 20% year to date.
OMGA’s earnings beat estimates in two of the trailing four quarters, met the mark in one and missed in another, delivering an average surprise of 8.24%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
ADC Therapeutics SA (ADCT) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
Omega Therapeutics, Inc. (OMGA) : Free Stock Analysis Report
